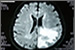
Glioblastoma multiforme (GBM) is the most common form of primary brain cancer. Every year, approximately 19,000 people are diagnosed with brain cancer. GBM is particularly difficult because it is resistant to many standard treatments, which include chemotherapy, radiation, and surgery. The Food and Drug Administration (FDA) has recently approved a new medical device for treating GBM. The NovoTTF-100A System is a portable machine that can be plugged into a wall or operated by batteries. Patients connect the device to their scalps and receive a series of low-intensity, alternating electrical fields. Although there is an increased incidence of headaches and convulsions associated with the machine, there is a decreased incidence of nausea, fatigue, anemia, and infection.
Currently, the device is not to be used with any other treatment, and it is intended only for patients who have failed all other treatments.
