Dalteparin
The FDA has approved dalteparin for the prevention and treatment of deep vein thrombosis and pulmonary embolism in cancer patients. Dalteparin is also approved to prevent deep vein thrombosis in high risk patients, including: Patients undergoing abdominal surgery or hip replacement surgery, patients with unstable angina or myocardial infarction, patients with restricted mobility due to an acute illness.1
- 1 Dalteparin. MedlinePlus. https://medlineplus.gov/druginfo/meds/a696006.html
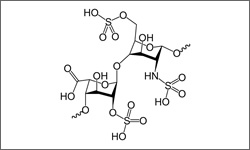
Dalteparin (Fragmin®) is a low molecular weight heparin that prevents clot formation by increasing the effects of anti-thrombin. Heparin is a variable-sized chain-like molecule. A small segment of the heparin backbone is shown in the image.
Due to an increased risk of bleeding, dalteparin should be used cautiously in patients also receiving oral anticoagulants, platelet inhibitors or thrombolytic drugs. Dalteparin is not recommended for cancer patients undergoing regional anesthesia or for those with allergies to heparin or pork products. Caution should be used in patients with an increased risk of hemorrhage as well as those with a history of heparin-induced platelet depletion. The potential for fetal harm in pregnant women treated with dalteparin has not been studied, therefore the drug should be used only when necessary and nursing mothers should exercise caution as there is some evidence that low levels of the drug can pass into breast milk.
