Imiquimod
Brand name:
Aldara®
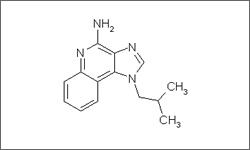
IUPAC:
3-(2-methylpropyl)-3,5,8-triazatricyclo[7.4.0.02,6]trideca-1(9),2(6),4,7,10,12-hexaen-7-amine
FDA approval:
Yes
Usage:
Imiquimod is supplied as a cream and applied externally to lesions. Conditions for which imiquimod is used include Superficial Basal Cell Carcinoma (BCC), Actinic Keratosis, External genital warts. Imiquimod is currently under investigation (in humans) for its effectiveness in treating other skin cancers, including melanoma.1
- 1 Imiquimod Topical. MedlinePlus. https://medlineplus.gov/druginfo/meds/a698010.html
Mechanism:
Imiquimod's (Aldara®) anti-cancer properties result from its ability to stimulate the body's own defenses (immune system) to destroy cancer cells. 1
The structure below is the 3D conformer Imiquimod.
- 1 Schon M, Schon MP. "The antitumoral mode of action of imiquimod and other imidazoquinolines." 2007. Current Medical Chemistry 14(6):681-687 [PUBMED]
