Epirubicin
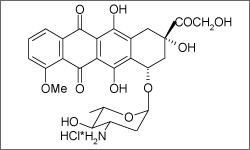
Epirubicin is used to treat breast cancers with lymph node involvement after the tumor is removed (resected), as well as metastatic gastric cancer. Epirubicin is administered as an intravenous infusion.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Epirubicin (a derivative of Doxorubicin) is an anthracycline antibiotic that affects cancer cells in two different ways.The drug wedges in DNA (intercalates). This stops the DNA from being copied (replication) and/or being used to make proteins (synthesis). The drug inhibits the activity of an enzyme, topoisomerase type II, which leads to breaks in the DNA.1
The molecule above shows the 3D conformer structure of Epirubicin.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects include nausea and vomiting, fatigue, hair loss, mouth sores. Epirubicin may cause blood cell counts to decrease. Patients should have blood tests before each treatment cycle to monitor this. Patients may also take an antibiotic during treatment to prevent infections. Epirubicin may cause more serious delayed side effects which include heart problems or acute myelogenous leukemia. However, these serious effects are rare.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Epirubicin should not be taken by women who are pregnant and patients should not become pregnant while using this drug, as it may have harmful affects on the developing fetus. This drug may have effects on fertility after treatment has ended.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
