Erlotinib
The FDA has approved erlotinib to treat: Locally advanced or metastatic non-small cell lung cancer (after failure of at least one prior therapy), as well as locally advanced, unresectable or metastatic pancreatic cancer (in combination with gemcitabine).1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
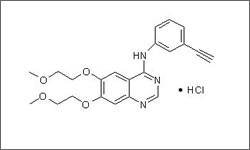
Erlotinib (Tarceva®) is a tyrosine kinase inhibitor associated with the human epidermal growth factor receptor (EGFR). By inhibiting the function of EGFR, which is overexpressed in cancer cells, erlotinib blocks cell proliferation and promotes death of cancer cells.1
The diagram below shows the 3D molecular structure of Erlotinib.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Side Effects include: Anemia and thrombocytopenia (low red and white blood cell counts), Diarrhea, anorexia, nausea and vomiting, Cough, Shortness of breath and Eye irritation. In addition to these, a very common side effect is pruritus, which is an intense skin rash that disperses negative effects throughout the whole body.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Potentially serious adverse reactions to erlotinib include pulmonary toxicity (including pneumonia, interstitial lung disease and acute respiratory distress syndrome), myocardial infarction, hemorrhage and liver failure. Due to the potential for harm to the fetus, women should not become pregnant during or immediately following erlotinib treatment. Women who are already pregnant should be warned of this potential danger to the developing fetus. New mothers are advised not to nurse infants during treatment since it is possible that erlotinib can pass into breast milk. CYP3A4 inhibitors and inducers change the metabolism of erlotinib and therefore special attention must be paid when the two treatments are administered simultaneously.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
