Gemcitabine
Malignancies for which gemcitabine is used include non-small cell lung cancer (in combination with cisplatin), pancreatic cancer (advanced or metastatic), advanced ovarian cancer (in combination with carboplatin). 1 Gemcitabine is administered as an intravenous infusion.
- 1 U.S. Food and Drug Administration: Office of Oncology Drug Products (accessed 2/4/08) [http://www.fda.gov/cder/Offices/OODP/whatsnew.htm]
Gemcitabine (Gemzar®) is an antimetabolite that acts as a pyrimidine analog. It is incorporated into a dividing cell's DNA which causes the cell to undergo apoptosis through triphosphate metabolite inhibition.1
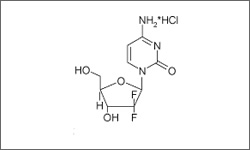
The diagram below shows the 3D molecular structure of Gemcitabine.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects include bone marrow suppression, weakness, fever, nausea and vomiting, rash. Side effects may be different or more severe if gemcitabine is being administered with other cancer treatments. Gemcitabine can be a suppressor of bone marrow activity. It is important to monitor blood cell counts throughout the duration of treatment and especially before each infusion. Gemcitabine may result in toxicities of other bodily systems including the hepatic (liver), renal (kidneys), and pulmonary (lungs) systems. For this reason the function of these systems should be monitored throughout treatment to prevent severe complications. Gemcitabine should not be taken by women who are pregnant and patients should not become pregnant while using this drug, as it may have harmful affects on the developing fetus.1
- 1 Gemzar.. Prescribing Information. Eli Lilly and Company. 2014. [http://www.gemzar.com]
