Lenalidomide
The FDA has approved lenalidomide for the treatment of: Multiple myeloma in patients who have received prior therapy (in combination with dexamethasone), transfusion-dependent anemia in patients with myelodysplastic syndromes who have a 5q deletion abnormality.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Lenalidomide (Revlimid®) action is not completely understood, but as an analog of thalidomide, this Immunomodulatory drug stimulates the proliferation of T-cells and other cells. Lenalidomide also promotes the inhibition of basic fibroblast growth (bFBG) and vascular endothelial growth factor (VEGF). Lenalidomide is also believed to have the ability to alter the immune system and destroy both cancer cells and their ability to recruit blood vessels.1
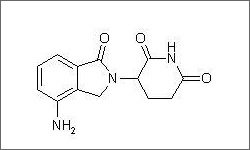
The diagram above shows the 3D molecular structure of Lenalidomide.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Side Effects include: Low blood counts (platelets, red blood cells and white blood cells), diarrhea, constipation and/or nausea, rash and itching, fatigue and perceived weakness, fever. There can also be severe teratogenic effects (pregnancy issues).1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Lenalidomide (as an analog of thalidomide) poses an extremely high risk to a developing fetus and can result in severe birth defects and even death. Therefore, lenalidomide is distributed via the RevAssist® program, in which patients, pharmacies and prescribing physicians much register. Before beginning lenalidomide treatment, a woman of childbearing age must have two negative pregnancy tests and be willing to employ two separate methods of birth control before, during and at least four weeks after treatment. Women must have repeated pregnancy tests throughout treatment. Males receiving lenalidomide must not have unprotected sexual intercourse with women of childbearing age during treatment since it is unknown whether the drug can be present in semen. Men cannot donate sperm, and neither men nor women can donate blood during lenalidomide treatment. Also, new mothers should discontinue nursing infants during and immediately following treatment.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
