Abemaciclib
Brand name:
Verzenio®
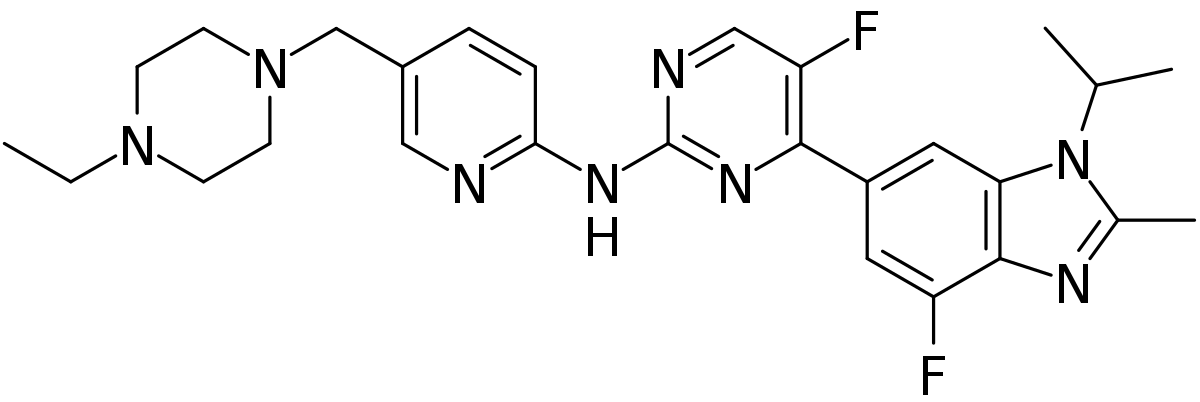
IUPAC:
N-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine
FDA approval:
Yes
Usage:
Verzenio is a prescription medicine used:
- In combination with fulvestrant to treat women with hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer or breast cancer that has spread to other parts of the body (metastatic), whose disease has progressed after hormonal therapy.
- Alone to treat adults with HR–positive, HER2–negative advanced breast cancer or metastatic breast cancer whose disease has progressed after hormonal therapy and prior chemotherapy.
Mechanism:
An orally available cyclin-dependent kinase (CDK) inhibitor that targets the CDK4 (cyclin D1) and CDK6 (cyclin D3) cell cycle pathway, with potential antineoplastic activity. Abemaciclib specifically inhibits CDK4 and 6, thereby inhibiting retinoblastoma (Rb) protein phosphorylation in early G1. 1
- 1 https://hemonc.org/wiki/Abemaciclib_(Verzenio)
Side effects:
The most common side effects of Verzenio include: nausea, infections, decreased appetite, headache, abdominal pain, tiredness and vomiting.
The most common changes to blood tests were low red blood cell count (anemia), low white blood cell counts (leukopenia) and low platelet count (thrombocytopenia).
