Hydroxyurea
Malignancies for which hydroxyurea is used include: Melanoma, Chronic myelogenous leukemia, essential thrombocytosis, Ovarian cancer (recurrent, metastatic, or inoperable) and Squamous cell carcinomas of the head and neck. Hydroxyurea is usually administered as an oral capsule.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Hydroxyurea (Hydrea®) is thought to inhibit DNA synthesis and prevent cell division, but does not interfere with the production of RNA or protein. Though the exact mechanism of action remains unknown, research suggests that DNA synthesis is blocked by interfering with the activity of an enzyme that is important for the process.1
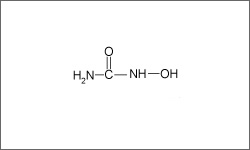
The diagram above shows the 3D molecular structure of Hydroxyurea.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects include: bone marrow depression, nausea and vomiting, diarrhea, constipation, and skin changes. Hydroxyurea should not be taken by women who are pregnant and patients should not become pregnant while using this drug, as it may have harmful affects on the developing fetus. 1
- 1 Hydrea.. Prescribing Information. Bristol-Myers Squibb Company. March, 2016. [http://packageinserts.bms.com/pi/pi_hydrea.pdf]
