Megestrol
Megestrol is used to treat recurrent, inoperable or metastatic breast or endometrial cancers and is administered in tablet form. Megestrol is also (although less frequently) used in treatment in renal cell cancer and as an appetite stimulant in cancer and HIV patients.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Megestrol (Megace®) is a synthetic analog of a naturally occurring bodily hormone called progesterone, which has antiestrogenic effects and metabolizes estrogen to less potent metabolites. Megestrol has the same effects in the body as progesterone in that it is growth inhibiting in hormonally sensitive cells that monitor estrogen receptor cells.1
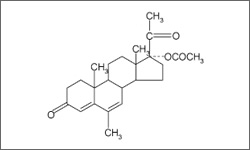
The diagram below shows the 3D molecular structure of Megestrol.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects for include weight gain due to increased appetite and mild fluid retention. Side effects may be different or more severe if megestrol is being administered with other cancer treatments. Some less common side effects of Megestrol use include: nausea and vomiting, hyperglycemia and heat flashes.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Megestrol must not be taken by women who are pregnant as it can cause harm to the unborn child. In diabetics, treatment with megestrol may result in a worsening of diabetes that results in an increase in insulin requirements.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
