Sonidegib
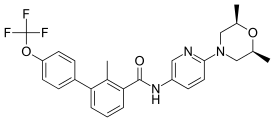
Odomzo® (sonidegib) was approved by the FDA in 2015. It is used to treat locally advanced basal cell carcinoma (BCC) in patients who cannot be treated with surgery or radiation therapy and in patients whose BCC has recurred after surgery or radiation therapy.
Sonidegib (Odomzo®) is a Hedgehog pathway inhibitor. Sonidegib inhibits the Hedgehog pathway by binding to and inhibiting Smoothened, a transmembrane protein involved in Hedgehog signaling. The hedgehog pathway is overactive is basal cell carcinoma (BCC), so suppression of this pathway may reduce and stop BCC growth.1
The diagram above shows the 3D molecular structure of Sonidegib.
- 1 Sonidegib. MedlinePlus. 2016. https://medlineplus.gov/druginfo/meds/a615034.html
Most common side effects include: muscle spasms, alopecia, dysgeusia, fatigue, nausea, musculoskeletal pain, diarrhea, weight loss, appetite loss, myalgia, abdominal pain, headache, pain, vomiting, and pruritus.1
- 1 Sonidegib. MedlinePlus. 2016. https://medlineplus.gov/druginfo/meds/a615034.html
