Trastuzumab
Herceptin® is used to treat metastatic and early-stage breast cancer and is also FDA-approved to treat metastatic gastric cancer in combination with other drugs. Trastuzumab (Herceptin®) is given both alone and in combination with other treatments. In many cases, combination treatment with chemotherapy has been found to yield better results. Herceptin® is given via intravenous infusion in the arm or hand.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
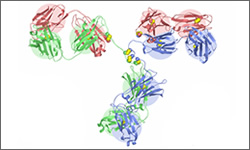
Trastuzumab binds to the extracellular segment of the epidermal growth factor receptor ( also called HER2, HER-2, HER2/neu). The binding blocks signals that would otherwise result in cell division. The result is a reduction in reproduction of cells treated with the drug. Note that the image shown is a general structure of an antibody. There are four proteins bound together in a 'Y' shape. The two top tips of the Y are where the antibody binds with its target. This means that each antibody molecule can bind to two identical target regions.1
The diagram above shows the 3D molecular structure of meditope-enabled Trastuzumab.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects associated with treatment with Trastuzumab are heavily infusion dependent, as they include: fever, chills, fatigue, nausea and vomiting. Less common side effects include: myelosuppression, cardiotoxicity and pulmonary toxicity.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
