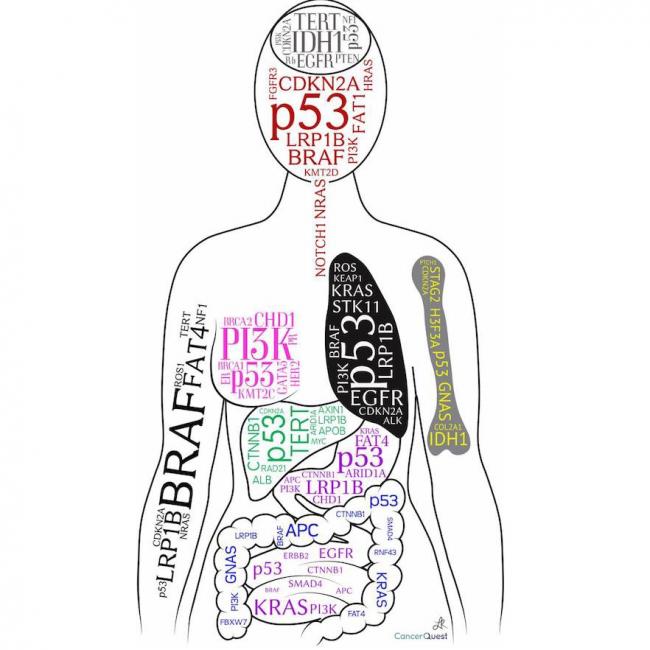
Introduction
The cell division process is dependent on a tightly controlled sequence of events. These events are dependent on the proper levels of transcription and translation of certain genes. When this process does not occur properly, unregulated cell growth may be the end result. Of the 30,000 or so genes that are currently thought to exist in the human genome, there is a small subset that seems to be particularly important in the prevention, development, and progression of cancer. These genes have been found to be either malfunctioning or non-functioning in many different kinds of cancer.
The genes that have been identified to date have been categorized into two broad categories, depending on their normal functions in the cell.
- Genes whose protein products stimulate or enhance the division and viability of cells. This first category also includes genes that contribute to tumor growth by inhibiting cell death.
- Genes whose protein products can directly or indirectly prevent cell division or lead to cell death.
The normal versions of genes in the first group are called proto-oncogenes. The mutated or otherwise damaged versions of these genes are called oncogenes. Note that by convention gene names are italicized and the proteins they make are not. As an example TP53 refers to the gene and p53 refers to the protein.
The genes in the second group are called tumor suppressors. Further information on the topics on this page can also be found in most introductory Biology textbooks, we recommend Campbell Biology, 11th edition.2
Topics on this page:
Oncogenes
A useful analogy to consider when thinking about tumor suppressors and oncogenes is an automobile. The proto-oncogenes would be in control of the movement of a car (the gas pedal in the animation below). When everything is functioning properly, the car moves only when the gas pedal is pushed. In normal cells, both internal and external signals control the activity of the oncogenes. In the animation below, these signals would be represented by the the 'X' shaped growth factor and the foot in the video portion.
A defective oncogene would be analogous to a gas pedal that is stuck in the 'on' position. There is no longer a need for signals to activate these genes. The car would go forward regardless of whether the pedal was pushed or not!
What this means for cells is that they divide continuously even in the absence of any signals telling them to divide. We have two copies of each gene and for oncogenes, a single defective copy is enough to cause a cell to divide.
Numerous genes have been identified as proto-oncogenes. Many of these genes are responsible for providing the positive signals that lead to cell division. Some proto-oncogenes work to regulate cell death. As stated in the introduction to this section, the defective versions of these genes, known as oncogenes, can cause a cell to divide in an unregulated manner. This growth can occur in the absence of normal pro-growth signals such as those provided by growth factors. A key feature of oncogene activity is that a single altered copy leads to unregulated growth. This is in contrast with tumor suppressor genes which must BOTH be defective to lead to abnormal cell division.
The proto-oncogenes that have been identified so far have many different functions in the cell. Despite the differences in their normal roles, these genes all contribute to unregulated cell division if they are present in a mutant (oncogenic) form. The mutant proteins often retain some of their capabilities but are no longer sensitive to the controls that regulate the normal form of the protein.
Different cancer types tend to depend on a limited number of 'driver' oncogene mutations. These mutations are the main changes that make the cancer progress. ALL cancers have lots of additional changes, the so-called 'passenger' mutations, that may contribute to the cancer, but are not the main genes.
Selected oncogenes that have been associated with numerous cancer types are described in more detail below.
HER2/Neu (ERBB2)
HER2 protein bound to the binding portion (Fab) of the Herceptin® antibody.
HER2/neu (also called ERBB2) is the gene that encodes the human epidermal growth factor receptor type 2. This receptor is found in moderate levels on some normal cells and as the gene's name implies, it is involved in cellular responses to growth factors. As shown below, binding of the growth factor can lead to cell division.
Extra copies of the HER2/neu gene are found in up to 20% of human breast cancers. The increase in the number of HER2/neu genes (called gene amplification) can lead to an increased amount of HER2 protein on the surface of the cells, and is thought to cause cell division (shown below).3 Gene amplification of HER2/neu is also thought to alter the response of tumors to treatment, as well as the ability of the tumor to grow and spread. Having too many active copies of the HER2/neu gene may make a tumor more aggressive, but may also make the cancer more sensitive to some chemotherapy agents.4More on gene amplification.
HER2/Neu and Cancer Treatments
Many studies have been conducted to define the role the HER2 protein plays in the response to chemotherapy. One study exposed the cells of 140 primary breast tumors to different amounts of two different chemotherapy combinations. Cells with lots of HER2/neu gene activity were more responsive to the chemotherapy treatments.5 A review of clinical trials extended this finding, identifying greater sensitivity to anthracyclin-based chemotherapies, such as doxorubicin, in breast cancer patients with strong HER2 gene activity.6 Chemotherapy attacks cells that are dividing, and HER2/neu amplification can stimulate cell division. One possible conclusion is that due to their high rate of cell division, cancer cells with high levels of HER2 are killed more effectively. While the overall effects of HER2/neu amplification vary, it remains a critical prognostic marker for breast cancer and can be a crucial guide for treatment.7
Historically, HER2/neu overactivity has been associated with more abnormal cancer cells (poor tumor cell differentiation), decreased patient survival, and a lack of hormone receptors (HR negative).8 Newer studies indicate that about half of HER2 positive breast cancers are actually HR positive. This presents challenges for targeted treatment.9 It is now clear that the HER2/neu proto-oncogene is important in the development and drug sensitivity of several forms of cancer but the story is far from complete.
Intrinsic Subtypes of Breast Cancer
While HER2 protein levels have been thoroughly studied as a biomarker for breast cancer, hormone receptor (HR) status – either estrogen receptor alpha (ERα) and/or progesterone receptor (PR) – is equally important for its value in predicting likely outcomes (prognoses/prognostic) and for guiding treatment choices.10 Motivated by the goal of finding additional prognostic factors, researchers have directed their focus on subsets of breast cancer with distinct gene activity (expression) patterns. Four main subpopulations consistently appear in genomic analysis of breast cancer tumors and are referred to as the intrinsic subtypes of breast cancer. Since first being recognized in 2000, the intrinsic subtypes have been repeatedly validated and include:11, 12
- Luminal A: high ER expression; less frequently mutated p53; less proliferative; best relative prognosis
- Luminal B: lower ER expression; more frequently mutated p53; more proliferative; more aggressive prognosis than Luminal A
- Basal-like: most frequently lack ER, PR, and HER2 (also known as Triple Negative Breast Cancer); high frequency of mutated p53; high proliferation
- HER2-enriched (HER2-E): high expression of HER-2/neu; high frequency of mutated p53; high proliferation
Luminal subtypes express genes associated with the secretory cells that line breast ducts. The gene expression pattern of the basal-like subtype is associated with that of cells that surround breast ducts. Interestingly, not all HER2-positive tumors correspond to the HER2-E subtype and not all HER2-E breast cancers produce too much HER2 protein.10 Despite the value of subtyping for disease prognosis, studies done to look for differences in responses to targeted treatment have not always produced clear results. Also, genomic analysis is often limited to recurrent or metastatic disease. Other tests, including immunohistochemistry (IHC), often produce more easily used results for less advanced breast cancers.13
Antibody treatment and HER2
Cancer treatments have been designed to combat cancers overexpressing the HER2 protein. Trastuzumab (Herceptin®), from Genentech, is a humanized monoclonal antibody that binds to the HER2 protein and blocks its activity, preventing excessive cell proliferation. This process is shown in the animation below. Herceptin® can be used with chemotherapy for HER2/neu amplified cancers.8, 14 More on antibody treatments for cancer.
RAS
The above structure is the H-RAS oncogene protein complexed to a hydrolysing GTP (GppNp).
RAS gene products are involved in kinase signaling pathways that control the transcription of genes, which then regulate cell growth and differentiation. To turn "on" the pathway, the RAS protein must bind to a particular molecule (GTP) in the cell. To turn the pathway "off," the RAS protein must break up the GTP molecule. Alterations in the RAS gene can change the RAS protein so that it is no longer able to break up and release the GTP. These changes can cause the the pathway to be stuck in the "on" position.15 The "on" signal leads to cell growth and proliferation. Therefore, RAS overexpression and amplification can lead to continuous cell proliferation, which is a major step in the development of cancer.16 Cell division is regulated by a balance of positive and negative signals. When ras transcription is increased, an excess of the gene's protein is in the cell, and the positive signals for cell division begin to outweigh the negative signals.
The conversion of RAS from a normal gene into an oncogene usually occurs through a point mutation in the gene. The altered function can affect the cell in different ways because RAS is involved in many signaling pathways that control cell division and cell death. Anti-cancer drugs are now being developed that target RAS dependent pathways. Much remains to be discovered before these drugs can be put into use.17
Mutant RAS has been identified in cancers of many different origins, including: pancreas (90%), colon (50%), lung (30%), thyroid (50%), bladder (6%), ovarian (15%), breast, skin, liver, kidney, and some leukemias.15 It is also possible that in the future, ras may be used to detect certain cancers. Historically, pancreatic cancer has been difficult to diagnose. The identification of RAS mutations in the DNA of pancreatic cells shed into feces may enable clinicians to differentiate between chronic pancreatitis and pancreatic cancer.15
MYC
The structure above shows MYC-Max recognizing DNA.
The MYC protein acts as a transcription factor and it controls the expression of several genes. Mutations in the MYC gene have been found in many different cancers, including Burkitt's lymphoma, B-cell leukemia, and lung cancer. The MYC family of oncogenes may become activated by gene rearrangement or amplification. Gene rearrangements involve the breakage and reunion of chromosomes. This process can involve large amounts of DNA and can affect many genes. The movement of a gene or group of genes to a different location within the same chromosome or to a different chromosome often leads to altered gene expression and cell function.
Translocation is one type of gene rearrangement, and a translocation between chromosomes 8 and 14 has been shown to result in overexpression of MYC and ultimately B-cell lymphoma. The animation below shows what a translocation between two different chromosomes looks like.
The amount of MYC protein present in the cell is important because the activity of MYC is balanced by another protein that opposes MYC activity. Therefore, an increase in either protein will offset the balance and affect cell division.18
In the video above, Dr. Gerard Evan discusses the role myc protein in cancer. Watch the full interview with Dr. Evan.
An increase in MYC activity is sometimes associated with programmed cell death, but this safeguard seems to be overridden in the presence of another oncogene, BCL2, which prevents MYC induced apoptosis.18
SRC
Above is the structure of human tyrosine kinase C-SRC.
SRC is the first oncogene ever discovered. It was identified as the transforming (cancer causing) agent of the Rous sarcoma virus (RSV), which infects chickens and other animals. RSV is a retrovirus. It infects cells and then inserts its own genes into the cellular DNA. This quickly results in the development of cancer. The virus is therefore called an acutely transforming virus. When infected, chickens develop large tumors within two weeks. Researchers discovered that the protein from a particular gene in RSV causes cells to grow in an abnormal manner. A corresponding proto-oncogene was found in the human genome. The human gene, when activated into an oncogene, functions in a similar manner.
The SRC protein is a tyrosine kinase. Kinases are enzymes that transfer phosphate groups onto target molecules. The important aspect of this process is that the removal/addition of phosphates changes biomolecules and is a key way by which the activities of cells are regulated. The phosphate addition/removal process acts like an on/off switch to control the activity of the target molecules. The src proteins alter several target molecules, resulting in the transmission of signals to the nucleus that help regulate the cell.18
There are at least nine different known SRC genes. Due to different processing of the mRNA produced by these genes, at least 14 different proteins may be produced. C-SRC is normally found in most cells at a low level, but have been found to be overexpressed in certain cancer types, including human neuroblastoma, small-cell lung, colon, and breast carcinomas, and rhabdomyosarcoma.15
Telomerase (gene symbol: TERT)
The structure above shows the RNA-binding domain of telomerase.
Due to the nature of the DNA replication process the ends (telomeres) of our chromosomes become shortened during each cell division. The shortening of the chromosomes serves to limit the number of times any given cell can undergo division. When telomeres shorten to a critical length the cell is unable to replicate its DNA without losing vital genetic material. At this point normal cells enter cellular senescence, or growth arrest, after which no further cell division takes place.
Cancer cells have the ability to replicate without reaching a state of senescence. In many cancers the ability to divide without limit is achieved by the production of an enzyme called telomerase. Telomerase maintains the ends of chromosomes so that they do not shorten. Telomerase is a normal protein that is present in cells during fetal development. In most cells of an adult human, telomerase is not present as the gene for the enzyme is not being expressed (transcribed and translated). However, in some cancer cells the necessary task of achieving unlimited replication is made possible by the reactivation of the gene that codes for telomerase.
The animation below depicts the lengths of chromosomes both with (right) and without (left) active telomerase.
In cancer cells that do not possess telomerase activity, shortening of chromosomes is thought to be prevented by other mechanisms. The maintenance of telomere length allows for unlimited cell division. The gene that codes for the active component of the telomerase enzyme, TERT, is considered a proto-oncogene because abnormal expression contributes to unregulated cell growth.
BCL2
The structure above is the antiapoptotic protein BCL-2.
BCL2 (for B cell lymphoma gene-2) proteins are associated with membranes and membrane activity. The BCL-2 protein is a part of a complex system of signaling that controls apoptosis. Apoptosis (cell death) may be induced by a variety of signals including irreparable DNA damage. This form of cellular suicide prevents the expansion of damaged cells. BCL-2 works to prevent apoptosis.19 Therefore, its overexpression can prevent apoptosis in cells that are damaged. This can lead to the continued division of the mutated cells lines and eventually cancer. Also, too much BCL-2 can contribute to metastasis in certain cancers.20
If apoptosis controls are disrupted, then drugs that work by inducing apoptosis will not work as efficiently. Therefore, drugs are being developed that will down-regulate BCL2 and allow other anti-cancer drugs to work more efficiently (and at lower doses). One such drug is the antisense nucleotide Genasense®, which was shown in phase I trials to reduce BCL-2 production. Subsequently, the company, Genta Pharmaceuticals, failed to obtain funding for additional research and was forced to close.21 More on antisense drugs.
Also, there are drugs that indirectly reduce the amount of BCL-2 protein, such as all-trans retinoic acid, paclitaxel, vincristine, and docetaxel. These drugs are are often combined with other chemotherapy agents during treatment. New methods, not yet tested in humans, include BCL-2 binding peptides that inactivate the protein and antimycin A that binds to BCL-2 related proteins. 22
Since BCL2 gene activity can affect the success of cancer treatment, knowing if it is functioning normally may prove to be a valuable diagnostic tool. This proto-oncogene can become activated into an oncogene by a translocation that causes overexpression of the gene, and an increased amount of BCL-2 protein has been discovered in many different types of cancers.23
EGFR
Oncogene Table
| Oncogene | Function/Activation | Cancer* | References |
| ABL1 | Promotes cell growth through tyrosine kinase activity | Chronic myelogenous leukemia | 26, 27 |
| AFF4/MLLT11 | Fusion affects the MLLT11 transcription factor/methyltransferase. MLLT11 is also called HRX, ALL1 and HTRX1 | Acute leukemias | 28, 29 |
| AKT2 | Encodes a protein-serine/threonine kinase | Ovarian cancer | 30, 31 |
| ALK | Encodes a receptor tyrosine kinase | Lymphomas | 32, 33 |
| ALK/NPM | Translocation creates fusion protein with nucleophosmin(npm) | Large cell lymphomas | 34, 35 |
| RUNX1 (AML1) | Encodes a transcription factor | Acute myeloid leukemia | 36, 37 |
| RUNX1/MTG8(ETO) | New fusion protein created by translocation | Acute leukemias | 38, 39 |
| AXL | Encodes a receptor tyrosine kinase | Hematopoietic cancers | 40, 41 |
| BCL-2, 3, 6 | Block apoptosis (programmed cell death) | B-cell lymphomas and leukemias | 42, 43 |
| BCR/ABL | New protein created by fusion of bcr and abl triggers unregulated cell growth | Chronic myelogenous and acute lymphotic leukemia | 44, 45 |
| MYC (c-MYC) | Transcription factor that promotes cell proliferation and DNA synthesis | Leukemia; breast, stomach, lung, cervical, and colon carcinomas; neuroblastomas and glioblastomas | 46, 47 |
| MCF2 (DBL) | Guanine nucleotide exchange factor | Diffuse B-cell lymphoma | 48 |
| DEK/NUP214 | New protein created by fusion | Acute myeloid leukemia | 49, 50 |
| TCF3/PBX1 | New protein created by fusion | Acute pre B-cell leukemia; TCF3 also called E2A | 51, 52 |
| EGFR | Cell surface receptor that triggers cell growth through tyrosine kinase activity | Squamous cell carcinoma, glioblastomas, lung cancer | 53, 54, 55 |
| MLLT11 | Fusion protein created by a translocation t(11;19). | Acute leukemias | 48, 28 |
| ERG/FUS | Fusion protein created by t(16:21) translocation. The erg protein is a transcription factor. | Myeloid leukemia | 56, 57 |
| ERBB2 | Cell surface receptor that triggers cell growth through tyrosine kinase activity; also known as HER2 or neu | Breast, salivary gland, and ovarian carcinomas | 58, 59 |
| ETS1 | Transcription factor | Lymphoma | 60, 61 |
| EWSR1/FLI1 | Fusion protein created by t(11:22) translocation. | Ewing Sarcoma | 62, 63 |
| CSF1R | Tyrosine kinase | Sarcoma | 64, 65 |
| FOS | Transcription factor for API | Osteosarcoma | 66, 67 |
| FES | Tyrosine kinase | Sarcoma | 68, 69 |
| GLI1 | Transcription factor | Glioblastoma | 70, 71 |
| GNAS (GSP) | Membrane associated G protein | Thyroid carcinoma | 72, 73 |
| HER2/neu | Overexpression of signaling kinase due to gene amplification | Breast and cervical carcinomas | 74, 7 |
| TLX1 | Transcription factor; aka HOX11 | Acute T-cell leukemia | 75, 76 |
| FGF4 | Encodes fibroblast growth factor; aka HST1 | Breast and squamous cell carcinomas | 48 |
| IL3 | Cell signaling molecule | Acute pre B-cell leukemia | 48 |
| FGF3 (INT-2) | Encodes a fibroblast growth factor | Breast and squamous cell carcinomas | 48 |
| JUN | Transcription factor for API | Sarcoma | 77, 16 |
| KIT | Tyrosine kinase | Sarcoma | 77, 16 |
| FGF4 (KS3) | Herpes virus encoded growth factor | Kaposi's sarcoma | 16 |
| K-SAM | Fibroblast growth factor receptor | Stomach carcinomas | 48 |
| AKAP13 | Guanine nucleotide exchange factor; aka LBC | Myeloid leukemias | 16, 78 |
| LCK | Tyrosine kinase | T-cell lymphoma | 16 |
| LMO1, LMO2 | Transcription factors | T-cell lymphoma | 16 |
| MYCL | Transcription factor | Lung carcinomas | 48, 77 |
| LYL1 | Transcription factor | Acute T-cell leukemia | 48 |
| NFKB2 | Transcription factor. Also called LYT-10 | B-cell lymphoma | 16 |
| NFKB2/Cα1 | Fusion protein formed by the (10;14)(q24;q32) translocation of NFKB2 next to the C alpha 1 immunoglobulin locus. | 48 | |
| MAS1 | Angiotensin receptor | Mammary carcinoma | 16 |
| MDM2 | Encodes a protein that inhibits and leads to the degradation of p53 | Sarcomas | 48, 77 |
| MLLT11 | Transcription factor/methyltransferase (also called HRX and ALL1) | Acute myeloid leukemia | 79, 16 |
| MOS | Serine/threonine kinase | Lung cancer | 48, 80 |
| RUNX1T1 | Fusion of transcription repressor to factor to a transcription factor. Also known as MTG8 and AML1-MTG8 | Acute leukemias | 48 |
| MYB | Transcription factor | Colon carcinoma and leukemias | 48 |
| MYH11/CBFB | New protein created by fusion of transcription factors via an inversion in chromosome 16. | Acute myeloid leukemia | 48 |
| NEU | Tyrosine kinase. Also called ERBB2 or HER2 | Glioblastomas, and squamous cell carcinomas | 81, 48 |
| MYCN | Cell proliferation and DNA synthesis | Neuroblastomas, retinoblastomas, and lung carcinomas | 81, 48 |
| MCF2L (OST) | Guanine nucleotide exchange factor | Osteosarcomas | 16 |
| PAX-5 | Transcription factor | Lympho-plasmacytoid B-cell lymphoma | 16 |
| PBX1/E2A | Fusion protein formed via t(1:19) translocation. Transcription factor | Acute pre B-cell leukemia | 48 |
| PIM1 | Serine/threonine kinase | T-cell lymphoma | 16 |
| CCND1 | Encodes cyclin D1. Involved in cell cycle regulation. Also called PRAD1 | Breast and squamous cell carcinomas | 48 |
| RAF1 | Serine/threonine kinase | Many cancer types | 48 |
| RARA/PML | Fusion protein caused by t(15:17) translocation. Retinoic acid receptor. | Acute premyelocytic leukemia | 81, 48 |
| HRAS | G-protein. Signal transduction. | Bladder carcinoma | 48 |
| KRAS | G-protein. Signal transduction | Lung, ovarian, and bladder carcinoma | 48, 77 |
| NRAS | G-protein. Signal transduction | Breast carcinoma | 48 |
| REL/NRG | Fusion protein formed by deletion in chromosome 2. Transcription factor. | B-cell lymphoma | 48, 77 |
| RET | Cell surface receptor. Tyrosine kinase | Thyroid carcinomas, multiple endocrine neoplasia type 2 | 81, 48 |
| RHOM1, RHOM2 | Transcription factors aka LMO1 and LMO2 | Acute T-cell leukemia | 48 |
| ROS1 | Tyrosine kinase | Sarcoma | 16 |
| SKI | Transcription factor | Carcinomas | 16 |
| SIS (aka PDGFB) | Growth factor | Glioma, fibrosarcoma | 16 |
| SET/CAN | Fusion protein formed by rearrangement of chromosome 9. Protein localization | Acute myeloid leukemia | 16, 82 |
| SRC | Tyrosine kinase | Sarcomas | 48, 83 |
| TAL1, TAL2 | Transcription factor.TAL1 is also called SCL | Acute T-cell leukemia | 48, 84 |
| NOTCH1 (TAN1) | Altered form of Notch (a cellular receptor) formed by t(7:9) translocation | Acute T-cell leukemia | 48, 85 |
| TIAM1 | Guanine nucleotide exchange factor | T-lymphoma | 16, 86 |
| TSC2 | GTPase activator | Renal and brain tumors | 16, 87 |
| NTRK1 | Receptor tyrosine kinase | Colon and thyroid carcinomas | 48, 88 |
* The cancer types listed in this column are those that are predominantly associated with each oncogene but this is not a complete list.
For information on these and other genes please visit the Cancer Genome Anatomy Project.
Section Summary: Oncogenes
Oncogenes
- Oncogenes are the mutated forms of normal cellular genes (proto-oncogenes).
- The protein products of proto-oncogenes stimulate cell division and/or inhibit cell death.
- Proto-oncogenes can be likened to the gas pedal in a car.
- Normally, internal and external signals strictly regulate the activity of the proto-oncogenes, but oncogenes are defective and are 'on' even when they do not receive appropriate signals.
- Oncogenes also help cells to ignore negative signals that would prevent a healthy cell from dividing.
- Oncogenes can cause cells to divide continuously even in the absence of any pro-growth signals.
- The following list describes different cellular roles for a few of the many known oncogenes:
- HER-2/neu
- HER-2/neu encodes for a cell surface receptor that can stimulate cell division
- The HER-2/neu gene is amplified in up to 30% of human breast cancers
- RAS
- The Ras gene products are involved in kinase signaling pathways that ultimately control transcription of genes, regulating cell growth and differentiation.
- Overexpression and amplification of RAS can lead to continuous cell proliferation.
- MYC
- The Myc protein is a transcription factor and controls expression of several genes.
- Myc is thought to be involved in avoiding the cell death mechanism.
- MYC oncogenes may be activated by gene rearrangement or amplification.
- SRC
- SRC was the first oncogene ever discovered.
- The Src protein is a tyrosine kinase which regulates cell activity.
- hTERT
- hTERT codes for an enzyme (telomerase) that maintains chromosome ends.
- In most normal cells telomerase is only present during fetal development.
- Activation of hTERT in adult cells gives them the ability to divide indefinitely.
- hTERT codes for an enzyme (telomerase) that maintains chromosome ends.
- BCL-2
- The Bcl-2 protein works to prevent cell death (apoptosis).
- Overexpression of BCL-2 allows continued division of mutated cells.
- HER-2/neu
Know the Flow: Oncogenes
Know the Flow is an educational game for you to test your knowledge. To play:
- Drag the appropriate choices from the column on the right and place them in order in the boxes on the left. Note that you will only use five of the six choices to complete the game.
- When done, click on 'Check' to see how many you got correct.
- For incorrect answers, click on 'Description' to review information about the processes.
- To try again, choose 'Reset' and start over.
Please visit us on a larger screen to play this game.
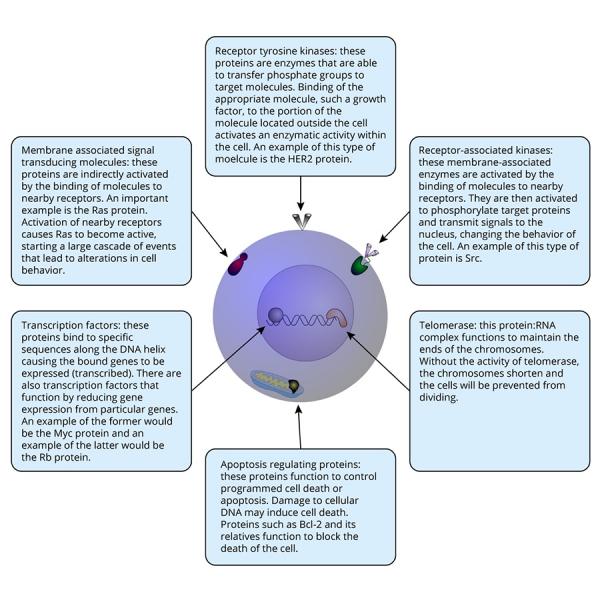
Look at the following diagram to see the functions of the oncogenes listed above. Many other oncogenes have activities similar to those shown.
Tumor Suppressors
Tumor suppressors function in many key cellular processes including the regulation of transcription, DNA repair and cell:cell communication. The loss of function of these genes leads to abnormal cellular behavior.
Continuing with the analogy from the previous page, tumor suppressors can be likened to the brake system in a car. If you think of each copy of any tumor suppressor gene as contributing some 'braking power' to the cell, then the analogy is reasonably good. When both copies of a tumor suppressor gene are functioning (represented below by the highlighed genes and stop signs) the cell can stop dividing (the car can stop moving).
A single defective tumor suppressor will still leave the cell with one functioning copy. It would be like stopping a car with only the rear or front brakes instead of both. It may not work quite as well, but it still works! The cells with a single defective version of a tumor suppressor can still control their cell division. When the second copy in the cell is lost, the cell loses the ability to prevent division.
A very good example of this principle will be discussed in the section on the retinoblastoma (Rb) tumor suppressor in the following pages.
All cancers demonstrate alterations in one or more tumor suppressors and oncogenes. In normal cells, these two groups of proteins work together to regulate cell division but in cancer cells the controls are no longer functioning properly.
Because these genes are so important to the development of cancer, the next few sections will examine some specific tumor suppressors and the cancers with which they are associated. The number of genes involved in these processes is increasing almost daily but the ones presented here are some of the best studied to date and should give a good introduction to some of the cellular functions that are disturbed in cancer.
Tumor Suppressors: TP53
The TP53 (often just written as p53) gene was discovered in 1979 and has emerged as one of the most important cancer-related genes to date. The gene, located on chromosome 17, produces a protein product that functions as a transcription factor . The genes controlled by p53 are involved in cell division and viability. Like the other tumor suppressors, the p53 protein functions to prevent unregulated cell growth.
The p53 protein interacts directly with DNA. It also interacts with other proteins that direct cellular actions. When DNA damage or other cellular insults are detected, p53 has the power to trigger cell death or apoptosis. The crucial role of p53 in maintaining proper control of cellular processes is underscored by the fact that the TP53 gene is found to be defective in about half of all tumors, regardless of their type or origin.18, 89 The mutations that inactivate TP53 may be acquired during the lifespan of an individual (sporadic mutations) or they may be inherited. transcription.
A Closer Look at the Discovery of TP53 Gene
In 1979, scientists discovered a novel protein. This protein, which could bind to a transforming protein (Large T antigen) from Simian Virus 40 (SV40), was more prevalent in cells transformed (immortalized and made potentially tumorigenic) by this virus than in normal cells. The protein and its corresponding gene were named p53, in reference to the mass of the protein (53 kilodaltons). The p53 gene is located on chromosome 17 at position p13.90
Although TP53 was the second tumor suppressor gene to be discovered (after RB1), scientists did not understand its true role in the cell until ten years after its discovery.18 Because p53 was present at increased levels in transformed cells, researchers first believed that it acted as an oncogene.91 This belief was supported by initial research. Scientists found that when the TP53 gene was transferred into cells, the cells underwent transformation. However, researchers later discovered that the TP53 gene that had been transferred was in fact a mutant form of the gene. One normal function of the TP53 gene is to prevent cell transformation!91, 18
Several lines of evidence have lead to the conclusion that this gene is a tumor suppressor. Since 1989, research into TP53 has made steady progress. The protein has been found to be involved in numerous cellular processes. However, there is still debate regarding the feasibility of using TP53 as a clinical tool, such as its use in identifying cancerous cells.92 Some current research efforts are designed to test treatments that repair the gene or replace it when it becomes damaged.93
p53 Function
The p53 protein plays an integral role in the cell and is normally present in all cell types. The protein is localized in the nucleus where it functions as a transcription factor. The p53 protein is at the center of a large network of proteins that 'sense' the health of a cell and cellular DNA. The p53 protein is the conductor of a well orchestrated system of cellular damage detection and control. When damage is sensed, the activity of the p53 protein aids in the decision between repair and the induction of cell death (apoptosis).94
As a transcription factor, p53 stimulates the transcription of a group of target genes. Among them, p21 is one of the most important. The product of the the p21 gene is a negative regulator of cyclin-dependent kinases, enzymes that are critical in the progression of the cell cyle and ultimately cell division.18 By stimulating the transcription of the p21 gene, p53 prevents cell proliferation. This stoppage gives the cell the opportunity to make repairs, if possible. If substantial DNA damage has occurred, the p53 protein can help to trigger cell death. The death of a cell that has incurred substantial DNA damage is beneficial to the organism because it prevents cells with deleterious mutations from proliferating.
As discussed in the introduction to this section, all cancer cells contain mutations in combinations of tumor suppressors and oncogenes. The removal of functional p53, the 'guardian of the genome,' from a cell allows for the accumulation of even more DNA damage and the division of cells that contain damaged DNA.
The mutation of the TP53 gene is one of the most frequent genetic changes seen in cancer cells. In addition to mutations that arise during the growth and development of individuals (sporadic mutations), there are forms of cancer associated with the inheritance of a damaged version of TP53. One such syndrome, the Li-Fraumeni cancer family syndrome, is associated with a wide variety of cancers.95 In addition, several viruses have evolved ways of inactivating the p53 protein including the human papillomavirus (HPV), the causative agent of cervical cancer.
Due to the central role played by this protein in the regulation of cell division, is a large amount of current research is committed to developing a safe method of restoring TP53 gene function.
In 2014, researchers discovered that an alternatively spliced form of p53 exists which works to DRIVE cancer development and spread. The new form is called p53Ψ. The work was done in cell culture as well as with mice and rabbit antibodies.96
A Closer Look at Abnormal p53 and Cancer Development
A cell lacking functional p53 may or may not become cancerous, and correspondingly, a cell with normal p53 function may eventually lead to the formation of a cancerous growth. As discussed in the section on Mutation, to become cancerous, several different changes to the DNA of a cell must occur. One of the functions of p53 is to monitor the status of the cell's DNA. Along with a host of additional proteins, p53 helps to recognize and effect repairs to damaged DNA. The responses to damaged DNA include repair, cessation of cell division and cell death. Damage to theTP53 gene does increase the likelihood of cancer development. Remember that since p53 is a tumor suppressor, both copies of the gene must be inactivated in order to see the full effects. There are several ways in which p53 can be inactivated:
Mutations
Alterations in the TP53 gene have have several different effects on the activity of the gene, depending on the location of the alteration.
-
Mutations may occur in regulatory regions. These portions of the gene control how often, and when, the gene is transcribed (this region is called the promoter). A mutation in the promoter region can result in a decrease or absence of p53 in the cell.97
-
Mutations that occur in the protein coding region of the gene can impact the expression of the gene (or activity of the protein) in several ways:
- A decrease in the activity of p53 as a transcription factor. The expression of the target genes of p53 that would be affected include p21 (a protein involved cell cycle regulation), Bax (a protein involved in the induction of apoptosis), and thrombospondin-1(an angiogenesis inhibitor).98, 99
- A change in p53 that makes it more susceptible to degradation. If the p53 proteins in the cell are being degraded at a higher-than-normal rate they will not be able to perform their functions as tumor suppressors.100
Viral Inactivation of p53
One of the functions of p53 is in 'guarding' the genome. Infection with viruses introduces foreign DNA into cells. p53, along with other proteins, is responsible for the cell's response to the presence of foreign DNA. Again, the responses include shutting down cell division and cell death. To avoid these responses, several different viruses have evolved ways of inactivating the p53 protein. An example of this is Simian Virus 40 (SV40) Upon infection with SV40, viral proteins are produced within the cell cytoplasm. One of the proteins produced is termed the Large T antigen. A function of this protein is the binding and inactivation the p53 protein. Other viruses, including hepatitis and human papillomavirus, produce similar proteins.
The elimination of functional p53 from the cell clears the way for cell division even in the presence of DNA damage. In the absence of p53, genetic instability as evidenced by increased mutations and aneuploidy are likely to increase. The increase in genetic damage leads to the accumulation of defective tumor suppressors and oncogenes.101
Overactivity of p53
While it may seem like a good idea to prevent cancer by somehow increasing the activity of p53, the outcomes are not always what one might expect. There have been several different studies looking into what would happen if p53 was always active.
In one, a mutant - always 'on' - version of p53 was put in mice. The good news is that the mice got much less cancer than normal animals. The bad news is that the animals seemed to age very quickly, developing curved spines, weight loss and other signs of old age. The animals died more quickly than unaltered animals. It seems that in this case having p53 active led to the death of too many cells, burning through the cellular lifespans very quickly.102 The results are still being examined, but provide a cautionary note.
In a 2022 study, p53 was kept active by removing the MDM2 gene from mice. MDM2 causes the inactivation of p53, so when it is gone, p53 can remain active. In thise animals, the continuous activity of p53 in the liver led to chronic inflammation and an increase in the development of liver cancer. The result was confirmed to be caused by p53, because when p53 was turned off, the animals were healthier again.103
Tumor Suppressors: The Retinoblastoma (RB1) Gene
The retinoblastoma gene (RB1) encodes a protein that acts by altering the activity of transcription factors. By interacting with transcription factors, Rb is able to indirectly control gene expression. In addition to this function, Rb and closely related proteins have several other less well documented activities. Ultimately, Rb and its relatives contribute to the control of the cell division process. 104
The RB1 gene is mutated in many types of cancer. One of the best studied is retinoblastoma, a cancer of the eye from which the gene got its name. The disease is often found in young children. Two different forms of retinoblastoma have been differentiated.
- The sporadic form of the disease can affect anyone and is dependent on genetic changes (mutations) aquired during the lifetime of the affected individual.
- The familial form of the disease results when affected individuals inherit a defective copy of the gene from one of their parents. In these individuals every cell contains one normal and one defective copy of the gene.105
As with other tumor suppressors, the cancer phenotype is not apparent unless both copies of the gene are damaged. While it is unlikely that the good copy of the RB1 gene will be mutated in any given cell, the enormous number of cells in our bodies (and even an eye) make it likely that the necessary second mutation will occur. Individuals with the inherited form of the disease often suffer from many different cancerous growths, especially osteosarcomas. Other types of cancer associated with RB1 mutation include lung, breast, and bladder carcinomas.106
A Closer Look at the RB1 Gene and its Association with Cancer
The RB1 gene was initially identified through its association with a familial (inherited) form of retinoblastoma. This type of cancer primarily affects the eyes and is most common in children. In its inherited form, affected individuals already have one mutated RB1 gene in all of their cells and require a single mutation in the other copy to render that particular cell devoid of functioning Rb protein. Mutations are rare events and the chance of a mutation in any particular gene is rather small but the extremely large number of cells in our bodies makes it very likely that the second copy of the gene will be damaged in at least a few cells. If these cells are then able to grow in an uncontrolled manner, cancer may ensue. In cases of familial retinoblastoma, it is common for affected individuals to develop multiple tumors because of the relatively high probability of this second mutation occurring. In its sporadic form, individuals normally have two functional copies of the RB1 gene in each of their cells and require two separate mutations in the same cell to lose Rb function. As a result, these individuals usually develop just one tumor. Individuals who have the familial form are much more likely to experience recurrences of tumors.
Loss of Rb activity has been identified in osteosarcomas found in patients with familial retinoblastoma. Osteosarcomas represent nearly half of the secondary tumors identified in patients with the inherited form of the disease. Rb function has also been shown to have an influence on a woman's chance of developing breast cancer. Normally Rb regulates the G1 cell cycle checkpoint, but studies have shown that some breast cancers have a deregulation in this checkpoint, implicating Rb as a contributing factor in the disease. Rb has been implicated as a contributor to some other types of cancers also, such as, small cell and non-small cell lung cancers.
Rb Function
The RB1 gene is essential for the normal functioning of the cell cycle. Cells respond to a variety of environmental factors that instruct them to grow and divide, rest, or undergo apoptosis. Disruption of these signals can lead to unregulated cell growth, which ultimately results in cancer. The control of the cell division process involves the integration of a variety of signals.
The RB1 gene product (pRb or Rb) normally functions as a growth inhibitor by binding to and inhibiting transcription factors. Therefore, Rb indirectly controls the expression of a variety of genes. Some of these genes produce proteins involved in driving cell division forward. Thus, Rb activity slows or halts cell division.107 Changes to regulatory proteins such as Rb can have dramatic effects on individual cells and ultimately the entire organism.Besides its involvement in regulation of the cell cycle, Rb also plays a role in apoptosis. Apoptosis is a very important cellular function in which a damaged cell undergoes programmed death. If a cell undergoes mutations that cannot be repaired, the cell can be eliminated via apoptosis. This process eliminates cells that have the potential for unregulated, potentially cancerous, growth. Any alterations in cellular function that reduce or eliminate the activation of apoptosis may have deleterious effects on the cell population.108 The RB1 gene may be inactivated via several different types of genetic damage. Mutations that completely eliminate the function of the protein (null mutations) are often seen in cells without functional Rb protein.
Loss of Rb may actually help patients respond to chemotherapy in some situations. Research has shown that breast cancer patients receiving neo-adjuvant chemotherapy, chemotherapy given before their surgery, respond better if they lack Rb function.109
A Closer Look at Rb's Functions
In addition to its role regulating cell growth and apoptosis, recent studies indicate that proteins related to Rb can have differential activity depending upon the stage of the cell cycle and their location within the nucleus. Further, some studies indicate that Rb-like proteins can regulate transcription of rRNA and tRNA, meaning that pRbs can exert control over both transcriptional and post-transcriptional events in cells.110
In addition to its role as a transcription factor, Rb has also proven to have additional activities that may contribute to its tumor suppressive effects. The Rb protein has been shown to associate with chromatin modification proteins such as histone deacetylases (HDAC). HDACs are thought to affect transcription by removing acetyl groups from histones. This modification results in a closer association between DNA and nucleosomes. 111 The closer DNA:histone interaction makes it more difficult for transcription( )factors like E2F to bind to their target regions in the DNA. It still remains unclear what Rb's function is in this process, but studies have shown that at least some HDACs do not function properly in the absence of Rb. 112
Tumor Suppressors: APC
Mutations of the APC (adenomatous polyposis coli) gene are strongly associated with both inherited and sporadic cases of colon cancer. As will be described in the next section, the APC protein, like many tumor suppressors, functions to control the expression of genes critical in the cell division process.
Most cases of colon cancer are thought to develop slowly over a period of several years. Inactivation of the APC gene, located on chromosome 5, is thought to lead to increased cell proliferation and contribute to the formation of colonic polyps. Several genetic alterations must occur during the conversion of normal colon cells into cells capable of forming tumors. In many cases mutation of the APC gene is thought to be one of the first steps. Evidence for this can be seen indirectly by examining individuals who have inherited a mutation in one of their APC genes. These patients have a disease called familial adenomatous polyposis, a condition in which the colon is filled with polyps. Every polyp has the potential to develop into cancer, therefore those with the inherited mutation are at a much higher risk for cancer. This situation is very similar to the one described for the inherited form of retinoblastoma in the section devoted to Rb. Instead of needing two somatic mutations within the same cell to lose APC function, these individuals require only a single genetic change (in any given cell) to cause major problems.
Comparisons of mutations identified in cells removed at different stages of cancer development have lead to the establishment of a possible order for genetic mutations that lead to a subset of colon cancers. In this model, the APC gene is mutated in the first step, producing highly proliferative cells. Those cells will then form a polyp, which may develop into cancer.18
APC Function
Since an absence of functional APC protein leads to increased cell division, it stands to reason that the normal protein works to inhibit cell division in some way. This is indeed the case. APC protein forms a complex with beta-catenin, a transcription factor, leading to beta-catenin's degradation. In the absence of the APC protein, there is an excess of beta-catenin in the nucleus. Beta-catenin binds to another protein in the nucleus to form a complex that binds to DNA and activates the transcription of several genes. One target gene of this complex is c-myc, a known oncogene.113C-myc is itself a transcription factor for several genes that control cell growth and division. The mutation of the APC gene leads, therefore, to a cascade of events that ultimately result in increased cell division. A diagram of this model of APC function is shown below.
Of course, many other factors can influence the expression of genes and their products, but mutations in the APC genes seem to correlate with an increase in beta-catenin and c-myc leading to high proliferation rates.114
Research has shown that the addition of normal APC protein to colon cancer cells lacking functional APC causes a decrease in tumor cell growth. The decrease in growth was shown to be caused by an increase in apoptosis, suggesting that APC mediates cell death controls as well as growth controls.115 Therefore, loss of the gene alters the balance between cell growth and cell death that acts to control cell numbers.
Tumor Suppressors: BRCA
The BRCA proteins have multiple functions. One important role is in the repair of DNA damage. They have also been implicated in the regulation of gene expression. The BRCA-1 gene is associated with the activation of another tumor suppressor, TP53, and its target gene p21 (also known as CDKN1A). BRCA proteins also interact with transcription factors and other transcription components to control the activity of several other genes.116 When the BRCA genes are non-functional, DNA repair and gene regulation are compromised. The increase in DNA damage can lead to the generation of cells that accumulate mutations in key genes, leading to cancer cell formation. Cells lacking functional BRCA genes often suffer from chromosomal breaks, severe aneuploidy, and may contain too many centrosomes. All of these defects interfere with normal cell division and cell function.
At the molecular level, the structure of the BRCA-1 and -2 genes provides an explanation for their susceptibility to mutation. They contain a very high proportion of repetitive DNA, which is rare in human genes. The repetitive DNA can lead to genomic instability and rearrangements. 116
Several lines of work have demonstrated that loss of BRCA gene products is associated with the development of sporadic and inherited cancer.117
A Closer Look at Ovarian Cancer and BRCA
Although the BRCA genes were named after their association with breast cancer, mutations in these genes are also associated with ovarian cancers. The hereditary and sporadic forms of ovarian cancer are similar but there are some differences. Hereditary ovarian cancer tends to have a mostly serous histology, be moderately to poorly differentiated, invasive, and is usually discovered at an advanced stage. Also, BRCA mutation carriers have a higher frequency of lesions in the fallopian tubes. Whether the patient is a carrier of the mutation or not, benign and low malignant potential ovarian tumors are not considered precursors of invasive ovarian carcinoma.118>
BRCA Function
Mutations in the BRCA-1 and BRCA-2 genes are associated with a subset of breast and ovarian cancers. These two genes have different functions within cells. Like the other tumor suppressors discussed so far, mutations can arise spontaneously or they may be inherited. Individuals who inherit a BRCA-1 or BRCA-2 mutation are known to be more susceptible to developing breast cancer. Individuals carrying a BRCA mutation have a lifetime risk (if they live to the age of 85) of 80% for developing breast cancer. The lifetime risks for developing ovarian cancer is 10-20% for BRCA-2 mutations and 40-60% for BRCA-1 mutations. The presence of these mutations may also increase the risk of prostate, pancreatic, colon, and other cancers.The total risk for any person depends on the individual genetic and environmental risk factors to which they are exposed. BRCA-1 and BRCA-2 mutations are thought to be associated with 5-10% of all breast cancers.
The BRCA Genes and Estrogen
Mutation of the BRCA genes has been associated with cancers of certain tissues, including the breast and ovaries. This suggests that estrogen may play a role in the development of cancer in these tissues. Estrogen fluctuations, such as those seen during puberty, menstruation, pregnancy, and menopause are associated with cancer development. Increases in estrogen, especially at puberty and during pregnancy, cause an increase in breast epithelial cell proliferation, which in turn places increased demands on the DNA repair capabilities of the cells. Reproduction (cell division) of cells with lowered DNA repair efficiency may lead to the formation of cancer. In the animation below, estrogen (shown in pink) stimulates cells to divide, producing a cancerous cell. 116
If one BRCA gene is already mutated, a mutation that removes the only functioning copy will cause DNA repair defects. When both copies of the repair gene are non-functional, there is an increased likelihood of a cell acquiring mutations that lead to tumor development. In an individual who has inherited a defective copy of the BRCA gene, ALL of their cells carry the defect. A mutation of the second copy in any cell can trigger DNA repair difficulties. Two independent mutation events are required for cancer development in individuals who have not inherited a defective BRCA allele. Both of these sporadic mutations must occur in the same cell. The occurrence of two mutations in the same cell is rare; this explains why these cancers tend to appear later in life.119
More information on this topic may be found in Chapters 3,4,7, and 9 of The Biology of Cancer by Robert A. Weinberg.
A Closer Look at BRCA's Affects on Survival
There have been several studies designed to determine the differences in survival between BRCA mutation carriers with cancer and those who developed cancer sporadically. The results are somehwhat contradictory, probably due to differing study designs and factors such as the degree of matching between controls (sporadic) and carriers. However, though the BRCA mutation carriers have a poorer prognosis based on the characteristics of their cancer, they seem to have an equal or higher survival rate when compared with sporadic cancer patients. This is thought to be due to the responsiveness of the tumors to chemotherapy. The increased susceptibility may be due, in part, to their high proliferation( )rates. The tumors are also more susceptible to cancer treatments such as gamma radiation, cisplatin, and mitomycin C because those treatments cause DNA damage that would normally be repaired by functioning BRCA gene products. If the BRCA genes are inactive the cell can not repair DNA damage as efficiently and cell death results. The non-cancerous cells in a BRCA mutation carrier retain one functional BRCA gene and can therefore repair their DNA.118
Tumor Suppressor Table
| Tumor Suppressor | Function | Cancer * | References |
| APC |
Controls the function of specific transcription factors which are involved in tumorigenesis, and development and homeostasis of some cell types including epithelial and lymphoid cells. APC has also been implicated in cell proliferation and other cellular activities such as migration, and adhesion.
|
Familial adenomatous and non-inherited colorectal carcinomas | 120, 121 |
| BRCA1, BRCA2 | DNA Damage Repair | Inherited breast cancers; ovarian cancers | 122 |
| CDKN2A | Gene locus that encodes the tumor suppressors p16 and p14ARF. | Brain tumors | 1 123 |
| DCC | Netrin-1 receptor. Regulation of cell proliferation and apoptosis of intestinal epithelium. | Colorectal carcinomas | 124, 125, 126 |
| DPC4 ( aka SMAD4) | Transciptional factor involved in development; Implicated in metastasis and tumor invasiveness. | Colorectal tumors, pancreatic neoplasia | 127, 128 |
| MADR2 (aka SMAD2) | Mediates signaling from growth factor receptors. Assists in transport of SMAD4 into nucleus. | Colorectal cancer | 129, 130 |
| MEN1 | Codes for the menin protein that interacts with transcription factors, DNA repair proteins, cytoskeletal proteins and others. Function not clearly defined. | Multiple endocrine neoplasia type 1 | 131 |
| CDKN2A (aka MTS1) | Inhibitor of cyclin-dependent kinases; regulates cell cycle passage from G1 into S. | Melanomas | 132 |
| NF1 | RAS GTPase activating protein (RAS-GAP) | Neurofibromatosis type 1 | 133 |
| NF2 | ERM protein; organize plasma membrane by assembling protein complexes and linking them to actin. | Neurofibromatosis type 2 | 134 |
| TP53 (often just p53 in older articles) | Encodes a transcription factor for p21, a protein that arrests the cell cycle in G1 phase. p53 integrates signals related to cell size, DNA integrity and chrommosome replication. | Bladder, breast, colorectal, esophageal, liver, lung, prostate, and ovarian carcinomas; brain tumors, sarcomas, lymphomas, and leukemias | 135 |
| PTEN | Lipid phosphatase. Regulates cell survival | Cowden syndrome; increased risk of breast and thyroid cancer | 2 136 |
| RB1 | Binds to, and inhibits, the E2F transcription factor. Halts cell cycle progression | Retinoblastoma, sarcomas; bladder, breast, esophageal, prostate, and lung carcinomas | 137 |
| VHL | Cell cycle regulation. May increase stability and activity of p53 | Renal cell carcinomas | 1 138 |
| WRN | DNA helicase and exonuclease. Involved in repair of DNA breaks. | Werner syndrome | 2 139 |
| WT1 | Transcription factor. Essential role in development. | Wilms tumors (pediatric kidney cancer) | 1 |
* The cancer types listed in this column are those that are predominantly associated with each tumor suppressor gene but this is not an exhaustive list.
For information on these genes and others please visit the Cancer Genome Anatomy Project.
1. Cooper G. Oncogenes. Jones and Bartlett Publishers, 1995.
2. Vogelstein B, Kinzler KW. The Genetic Basis of Human Cancer. McGraw-Hill: 1998.
Section Summary: Tumor Suppressors
Tumor Suppressors
- The protein products of tumor suppressor genes can directly or indirectly prevent cell division or lead to cell death.
- Tumor suppressors can be likened to the brake system in a car.
- Loss of function of tumor suppressors leads to abnormal cellular behavior.
- The following describes the function of some key tumor suppressor genes:
- p53
- A transcription factor that regulates genes controlling cell division and cell death.
- Important in the cellular response to DNA damage.
- Aids in decision between repair and induction of cell death.
- Functions by altering transcription factor activity.
- Contributes to the control of cellular division by acting as an inhibitor.
- The APC protein binds and stimulates the degradation of a transcription factor.
- Absence of functional APC protein leads to increased cell division.
- BRCA proteins have multiple functions including repairing DNA damage and regulation of gene expression.
- Non-functional BRCA leads to compromised DNA repair and gene regulation.
- p53
MicroRNAs
Genes are long strings of DNA that encode messages in the form of RNA. The genes of most interest, for many years, were those that encode messenger RNA (mRNA), the RNA that is used to guide the production of proteins. (See our Gene Function section for an overview.) Other RNAs (tRNA, snoRNA, rRNA) are useful at RNA (they are not used to make proteins). They work to help in the production of proteins.
In 1993, a new type of RNA was discovered in a type of worm- a very short RNA with surprising activities.140 The RNA was shown to regulate the activity of a different gene. While mRNA molecules can be thousands of nucleotides in length, the new RNA was only a few dozen nucleotides long. Within a decade, many other examples of these small RNAs were discovered. These microRNAs (or miRNAs) are now known to control many different genes and cellular processes.
Another surprise came when researchers looked for new miRNAs. Some of them come from their own gene, but many are found within other genes, usually in parts that are not used to make protein (so-called non-coding regions). Also, the miRNAs don't get produced in a functional form. They require several processing steps and ultimately work together with proteins to accomplish their gene regulating activities.
Two different pathways that lead to the production of functional miRNAs are shown in the diagram below (graphic from Wikimedia Commons).
The final product of the 'maturation' process is a short RNA combined with a group of proteins (a miRNP). The miRNPs work to increase and decrease the activity of target genes. The mature miRNPs can bind to target mRNAs and prevent them from being used to make protein. They can also directly cause the destruction of targets. Because the activity of our genes is very tightly controlled to maintain balance in our cells, it is not surprising that defects in miRNA production or activity have been linked to several human disorders, including cancer. 141, 142, 143, 144
MicroRNAs and Cancer
MicroRNAs (miRNAs) are now known to be extremely common and known to regulate genes that are involved in a wide range of cell activities. Changes in miRNAs that make them more or less active can influence the activity of their target genes and lead to visible changes, including disease. Cancer is the result of genetic changes that alter gene activity, so it make sense that changes in miRNAs could influence the development and/or spread of cancer. In fact, research on miRNAs is extremely active and is impacting many different areas of cancer biology, detection, diagnosis and treatment.145, 146, 147, 148 Some areas of cancer impacted by miRNAs are described below.
miRNAs and cancer prevention
Chemicals found in food are known to influence the activity of many genes, including those that encode miRNAs. The ways that our diet influences miRNA activity and could therefore increase or decrease cancer risk is currently an active area of investigation.149
miRNAs as oncogenes and tumor suppressors
Because miRNA control the activity of genes, they can be considered oncogenes or tumor suppressors, depending on their effects on cell growth. miRNAs that normally slow cell division or cause cell death would be considered tumor suppressors (their loss would lead to increased cell division/survival) and those that normally increase cell division or cell survival would be considered oncogenes. There are now many instances of miRNAs working in these ways, in a variety of different cancer types.143, 150, 144, 151
miRNAs as drivers of tumor cell metabolism
For many years, it has been known that cancer cells rely more on the energy production pathway called glycolysis than normal cells. This is referred to as the 'Warburg effect'. One result of this is that cancer cells take up more sugar than other cells and may be influenced by sugar levels in the body. Another impact is that the cancer cells produce more lactic acid, a product of glycolysis. This acid can change the enviroment around the cells. Together, the changes can lead to progression of the disease. It is now thought that miRNAs have a role in causing the Warburg effect by influencing the activity of tumor suppressors like p53 and oncogenes including HIF1A.152, 153, 154
miRNA as biomarkers for cancer detection and diagnosis
A biomarker is something that indicates the presence of a disease (or the potential for a disease) indirectly. An example of of a biomarker is the measurment of blood cholesterol levels as an indicator of cardiovasular health. Blood tests like the PSA test are also tests for biomarkers. Now that miRNAs have been associated with cancer, researchers are looking to see if the presence of miRNA in the blood or other tissues can serve as a biomarker of cancer and could therefore be the basis for a test.155, 146, 147, 148 They have also been proposed as markers of drug resistance and could be used to guide treatment.156
miRNA as targets for cancer treatment
As regulators of cell activities, miRNA should be possible targets for cancer treatments. Because a single miRNA can control a large group of genes, drugs targeted at miRNA could prove to be very effective. They could shut down or turn on entire pathways at once.157, 146, 147, 148, 150
An example is found in breast cancer. Many breast cancers are dependent on the female sex hormones estrogen and progestin for their growth and survival. This observation is actually the basis for the use of anti-hormonal treatments like tamoxifen, raloxifene and the aromatase inhibitors. In a 2012 study, progestin was shown to cause the cancer cells to revert to a more stem cell-like state, making them more difficult to treat. The change in cellular behavior was found to be due to the suppression of a group of miRNAs, known as the miRNA 29 family. The researchers are now looking at ways to increase the activity of these miRNAs in cancer cells in the hopes of reversing the stem cell traits in the cancer cells.158
miRNA as drivers of drug resistance
Sometimes, cancer drugs fight cancer effectively, but only at first. With time, a patient loses sensitivity to the drug. This phenomenon of drug resistance is what makes treating cancer so challenging. So, much of cancer research investigates why and how this resistance develops, and more and more evidence indicates that miRNA help drive this change in drug sensitivity. Abnormal levels of many miRNAs have been associated with drug resistance. However, when one of these miRNAs was restored to a normal level, sensitivity to a cancer drug returned.159
Learn more about stem cells and cancer.
- 1 Graphic created by Lauren Rusnak - 2017
- 2 Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., & Reece, J. B. (2017). Campbell Biology (11th ed.). Pearson.
- 3 Escrivá-de-Romaní, S., Arumí, M., Bellet, M., & Saura, C. (2018). HER2-positive breast cancer: Current and new therapeutic strategies. Breast (Edinburgh, Scotland), 39, 80–88. http://doi.org/10.1016/j.breast.2018.03.006 (Original work published June 2018) [PUBMED]
- 4 Cejalvo, J., Pascual, T., Fernández-Martínez, A., Brasó-Maristany, F., Gomis, R., Perou, C., … Prat, A. (2018). Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treatment Reviews, 67, 63–70. http://doi.org/10.1016/j.ctrv.2018.04.015 (Original work published June 2018) [PUBMED]
- 5 Konecny, G., Fritz, M., Untch, M., Lebeau, A., Felber, M., Lude, S., … Pegram, M. (2001). HER-2/neu overexpression and in vitro chemosensitivity to CMF and FEC in primary breast cancer. Breast Cancer Research and Treatment, 69(1), 53–63. (Original work published September 2001) [PUBMED]
- 6 Ding, W., Li, Z., Wang, C., Dai, J., Ruan, G., & Tu, C. (2018). Anthracycline versus nonanthracycline adjuvant therapy for early breast cancer: A systematic review and meta-analysis. Medicine, 97(42), e12908. http://doi.org/10.1097/MD.0000000000012908 (Original work published October 2018) [PUBMED]
- 7ab Nicolini, A., Ferrari, P., & Duffy, M. (2018). Prognostic and predictive biomarkers in breast cancer: Past, present and future. Seminars in Cancer Biology, 52(Pt 1), 56–73. http://doi.org/10.1016/j.semcancer.2017.08.010 (Original work published December 2018) [PUBMED]
- 8ab Spizzo G, Obrist P, Ensinger C, Theurl I, Dunser M, Ramoni A, Gunsilius E, Eibl G, Mikuz G, Gastl G. "Prognostic Significance of Ep-CAM and Her-2/neu Overexpression in Invasive Breast Cancer." Int. J. Cancer (2002). 98: 883-888. [PUBMED]
- 9 Schedin, T., Borges, V., & Shagisultanova, E. (2018). Overcoming Therapeutic Resistance of Triple Positive Breast Cancer with CDK4/6 Inhibition. International Journal of Breast Cancer, 2018, 7835095. http://doi.org/10.1155/2018/7835095 (Original work published December 2018) [PUBMED]
- 10ab Godoy-Ortiz, A., Sanchez-Muñoz, A., Parrado, M., Álvarez, M., Ribelles, N., Dominguez, A., & Alba, E. (2019). Deciphering HER2 Breast Cancer Disease: Biological and Clinical Implications. Frontiers in Oncology, 9, 1124. http://doi.org/10.3389/fonc.2019.01124 (Original work published December 2019) [PUBMED]
- 11 Perou, C., Sørlie, T., Eisen, M., van de Rijn, M., Jeffrey, S., Rees, C., … Botstein, D. (2000). Molecular portraits of human breast tumours. Nature, 406(6797), 747–52. (Original work published August 2000) [PUBMED]
- 12 Network, C. (2012). Comprehensive molecular portraits of human breast tumours. Nature, 490(7418), 61–70. http://doi.org/10.1038/nature11412 (Original work published October 2012) [PUBMED]
- 13 Geiersbach, K., Chen, H., Emmadi, R., Haskell, G., Lu, X., Liu, Y., & Swisshelm, K. (2020). Current concepts in breast cancer genomics: An evidence based review by the CGC breast cancer working group. Cancer Genetics, 244, 11–20. http://doi.org/10.1016/j.cancergen.2020.02.002 (Original work published February 2020) [PUBMED]
- 14 Tsuda H, Akiyama F, Terasaki H, Hasegawa T, Kurosumi M, Shimadzu M, Yamamori S, Sakamoto G. "Detection of Her-2/neu (c-erb B-2) DNA Amplification in Primary Breast Carcinoma." Cancer (2001). 92(12): 2965-2974. [PUBMED]
- 15abcd Ruddon RW. Cancer Biology. Oxford University Press: New York, 1995.
- 16abcdefghijklmnopqrs Vogelstein B, Kinzler KW. The Genetic Basis of Human Cancer. McGraw-Hill: 1998.
- 17 Ahmadian MR. "Prospects for Anti-ras Drugs." British Journal of Haematology (2002). 116(3-I): 511-518. [PUBMED]
- 18abcdefgh Cooper G. Oncogenes. Jones and Bartlett Publishers, 1995. 151-152, 175-176.
- 19 Strasser A, Huang DC, Vaux DL. "The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy." Biochim Biophys Acta. 1997 Oct 24;1333(2):F151-78. [PUBMED]
- 20 Fernandez Y, Gu B, Martinez A, Torregrosa A, Sierra A. "Inhibition of Apoptosis in Human Breast Cancer Cells: Role in Tumor Progression to the Metastatic State." Int. J. Cancer (2002). 101: 317-326. [PUBMED]
- 21 [View the Wikipedia page on Genta Pharmaceuticals]
- 22 Obasaju C, Hudes GR. "Paclitaxel and docetaxel in prostate cancer." Hematol Oncol Clin North Am. 2001 Jun;15(3):525-45. [PUBMED]
- 23 Gross A. "BCL-2 proteins: regulators of the mitochondrial apoptotic program." IUBMB Life. 2001 Sep-Nov;52(3-5):231-6. [PUBMED]
- 24abc Santos, Gilda Da Cunha, Frances A. Shepherd, and Ming Sound Tsao. "EGFR Mutations and Lung Cancer." Annual Review of Pathology: Mechanisms of Disease 6 (2011): 49-69. [http://www.ncbi.nlm.nih.gov/pubmed/20887192] [PUBMED]
- 25 Seshacharyulu, Parthasarathy et al. ¿Targeting the EGFR Signaling Pathway in Cancer Therapy.¿ Expert Opinion on Therapeutic Targets 16.1 (2012): 15¿31. PMC. [http://www.ncbi.nlm.nih.gov/pubmed/22239438] [PUBMED]
- 26 Gale, R., & Apperley, J. (2019). What Does Chronic Myeloid Leukaemia Tell Us About Other Leukaemias? Current Hematologic Malignancy Reports, 14(6), 477–479. http://doi.org/10.1007/s11899-019-00555-3 (Original work published December 2019) [PUBMED]
- 27 Cumbo, C., Anelli, L., Specchia, G., & Albano, F. (2020). Monitoring of Minimal Residual Disease (MRD) in Chronic Myeloid Leukemia: Recent Advances. Cancer Management and Research, 12, 3175–3189. http://doi.org/10.2147/CMAR.S232752 (Original work published December 2020) [PUBMED]
- 28ab Marschalek, R. (2016). Systematic Classification of Mixed-Lineage Leukemia Fusion Partners Predicts Additional Cancer Pathways. Annals of Laboratory Medicine, 36(2), 85–100. http://doi.org/10.3343/alm.2016.36.2.85 (Original work published March 2016) [PUBMED]
- 29 Meyer, C., Burmeister, T., Gröger, D., Tsaur, G., Fechina, L., Renneville, A., … Marschalek, R. (2018). The MLL recombinome of acute leukemias in 2017. Leukemia, 32(2), 273–284. http://doi.org/10.1038/leu.2017.213 (Original work published December 2018) [PUBMED]
- 30 Zheng, B., Geng, L., Zeng, L., Liu, F., & Huang, Q. (2018). AKT2 contributes to increase ovarian cancer cell migration and invasion through the AKT2-PKM2-STAT3/NF-κB axis. Cellular Signalling, 45, 122–131. http://doi.org/10.1016/j.cellsig.2018.01.021 (Original work published December 2018) [PUBMED]
- 31 Linnerth-Petrik, N., Santry, L., Moorehead, R., Jücker, M., Wootton, S., & Petrik, J. (2016). Akt isoform specific effects in ovarian cancer progression. Oncotarget, 7(46), 74820–74833. http://doi.org/10.18632/oncotarget.11204 (Original work published November 2016) [PUBMED]
- 32 Singh, A., & Chen, H. (2020). Optimal Care for Patients with Anaplastic Lymphoma Kinase (ALK)-Positive Non-Small Cell Lung Cancer: A Review on the Role and Utility of ALK Inhibitors. Cancer Management and Research, 12, 6615–6628. http://doi.org/10.2147/CMAR.S260274 (Original work published December 2020) [PUBMED]
- 33 Kong, X., Pan, P., Sun, H., Xia, H., Wang, X., Li, Y., & Hou, T. (2019). Drug Discovery Targeting Anaplastic Lymphoma Kinase (ALK). Journal of Medicinal Chemistry, 62(24), 10927–10954. http://doi.org/10.1021/acs.jmedchem.9b00446 (Original work published December 2019) [PUBMED]
- 34 Fuchs, S., Naderi, J., & Meggetto, F. (2019). Non-Coding RNA Networks in ALK-Positive Anaplastic-Large Cell Lymphoma. International Journal of Molecular Sciences, 20(9). http://doi.org/10.3390/ijms20092150 (Original work published April 2019) [PUBMED]
- 35 Cao, Z., Gao, Q., Fu, M., Ni, N., Pei, Y., & Bin Ou, W.-. (2019). Anaplastic lymphoma kinase fusions: Roles in cancer and therapeutic perspectives. Oncology Letters, 17(2), 2020–2030. http://doi.org/10.3892/ol.2018.9856 (Original work published February 2019) [PUBMED]
- 36 Jalili, M., Yaghmaie, M., Ahmadvand, M., Alimoghaddam, K., Mousavi, S., Vaezi, M., & Ghavamzadeh, A. (2018). Prognostic Value of RUNX1 Mutations in AML: A Meta-Analysis. Asian Pacific Journal of Cancer Prevention : APJCP, 19(2), 325–329. (Original work published February 2018) [PUBMED]
- 37 Rossetti, S., & Sacchi, N. (2013). RUNX1: A microRNA hub in normal and malignant hematopoiesis. International Journal of Molecular Sciences, 14(1), 1566–88. http://doi.org/10.3390/ijms14011566 (Original work published January 2013) [PUBMED]
- 38 van der Kouwe, E., & Staber, P. (2019). RUNX1-ETO: Attacking the Epigenome for Genomic Instable Leukemia. International Journal of Molecular Sciences, 20(2). http://doi.org/10.3390/ijms20020350 (Original work published January 2019) [PUBMED]
- 39 Lin, S. (2017). RUNX1-ETO Leukemia. Advances in Experimental Medicine and Biology, 962, 151–173. http://doi.org/10.1007/978-981-10-3233-2_11 (Original work published December 2017) [PUBMED]
- 40 Antony, J., & Huang, R. (2017). AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Research, 77(14), 3725–3732. http://doi.org/10.1158/0008-5472.CAN-17-0392 (Original work published December 2017) [PUBMED]
- 41 Colavito, S. (2020). AXL as a Target in Breast Cancer Therapy. Journal of Oncology, 2020, 5291952. http://doi.org/10.1155/2020/5291952 (Original work published December 2020) [PUBMED]
- 42 Fletcher, L., Nabrinsky, E., Liu, T., & Danilov, A. (2020). Cell Death Pathways in Lymphoid Malignancies. Current Oncology Reports, 22(1), 10. http://doi.org/10.1007/s11912-020-0874-3 (Original work published January 2020) [PUBMED]
- 43 Klanova, M. (2020). BCL-2 Proteins in Pathogenesis and Therapy of B-Cell Non-Hodgkin Lymphomas. Cancers, 12(4). http://doi.org/10.3390/cancers12040938 (Original work published April 2020) [PUBMED]
- 44 Vetrie, D., Helgason, V., & Copland, M. (2020). The leukaemia stem cell: similarities, differences and clinical prospects in CML and AML. Nature Reviews. Cancer, 20(3), 158–173. http://doi.org/10.1038/s41568-019-0230-9 (Original work published December 2020) [PUBMED]
- 45 Shi, T., Huang, X., Zhu, L., Li, X., Li, L., & Ye, X. (2020). Adult Ph-positive acute lymphoblastic leukemia-current concepts in cytogenetic abnormalities and outcomes. American Journal of Cancer Research, 10(8), 2309–2318. (Original work published December 2020) [PUBMED]
- 46 Baluapuri, A., Wolf, E., & Eilers, M. (2020). Target gene-independent functions of MYC oncoproteins. Nature Reviews. Molecular Cell Biology, 21(5), 255–267. http://doi.org/10.1038/s41580-020-0215-2 (Original work published December 2020) [PUBMED]
- 47 Fatma, H., & Siddique, H. (2020). Role of long non-coding RNAs and MYC interaction in cancer metastasis: A possible target for therapeutic intervention. Toxicology and Applied Pharmacology, 399, 115056. http://doi.org/10.1016/j.taap.2020.115056 (Original work published December 2020) [PUBMED]
- 48abcdefghijklmnopqrstuvwxyz Cooper G. Oncogenes. Jones and Bartlett Publishers, 1995.
- 49 Sandén, C., & Gullberg, U. (2015). The DEK oncoprotein and its emerging roles in gene regulation. Leukemia, 29(8), 1632–6. http://doi.org/10.1038/leu.2015.72 (Original work published August 2015) [PUBMED]
- 50 Mendes, A., & Fahrenkrog, B. (2019). NUP214 in Leukemia: It’s More than Transport. Cells, 8(1). http://doi.org/10.3390/cells8010076 (Original work published December 2019) [PUBMED]
- 51 Organista-Nava, J., Gómez-Gómez, Y., Illades-Aguiar, B., & Leyva-Vázquez, M. (2016). Regulation of the miRNA expression by TEL/AML1, BCR/ABL, MLL/AF4 and TCF3/PBX1 oncoproteins in acute lymphoblastic leukemia (Review). Oncology Reports, 36(3), 1226–32. http://doi.org/10.3892/or.2016.4948 (Original work published September 2016) [PUBMED]
- 52 Hein, D., Borkhardt, A., & Fischer, U. (2020). Insights into the prenatal origin of childhood acute lymphoblastic leukemia. Cancer Metastasis Reviews, 39(1), 161–171. http://doi.org/10.1007/s10555-019-09841-1 (Original work published December 2020) [PUBMED]
- 53 Boeckmann, L. (2020). Molecular Biology of Basal and Squamous Cell Carcinomas. Advances in Experimental Medicine and Biology, 1268, 171–191. http://doi.org/10.1007/978-3-030-46227-7_9 (Original work published December 2020) [PUBMED]
- 54 Rutkowska, A., Stoczyńska-Fidelus, E., Janik, K., Włodarczyk, A., & Rieske, P. (2019). EGFR: An Oncogene with Ambiguous Role. Journal of Oncology, 2019, 1092587. http://doi.org/10.1155/2019/1092587 (Original work published December 2019) [PUBMED]
- 55 Velazquez, A. (2020). Tumor evolution in epidermal growth factor receptor mutated non-small cell lung cancer. Journal of Thoracic Disease, 12(5), 2896–2909. http://doi.org/10.21037/jtd.2019.08.31 (Original work published May 2020) [PUBMED]
- 56 Noort, S., Zimmermann, M., Reinhardt, D., Cuccuini, W., Pigazzi, M., Smith, J., … Zwaan, M. (2018). Prognostic impact of t(16;21)(p11;q22) and t(16;21)(q24;q22) in pediatric AML: a retrospective study by the I-BFM Study Group. Blood, 132(15), 1584–1592. http://doi.org/10.1182/blood-2018-05-849059 (Original work published December 2018) [PUBMED]
- 57 Zerkalenkova, E., Panfyorova, A., Kazakova, A., Baryshev, P., Shelihova, L., Kalinina, I., … Olshanskaya, Y. (2018). Molecular characteristic of acute leukemias with t(16;21)/FUS-ERG. Annals of Hematology, 97(6), 977–988. http://doi.org/10.1007/s00277-018-3267-z (Original work published June 2018) [PUBMED]
- 58 Kreutzfeldt, J., Rozeboom, B., Dey, N., & De, P. (2020). The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. American Journal of Cancer Research, 10(4), 1045–1067. (Original work published December 2020) [PUBMED]
- 59 Robichaux, J., Elamin, Y., Vijayan, R., Nilsson, M., Hu, L., He, J., … Heymach, J. , V. (2019). Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell, 36(4), 444–457.e7. http://doi.org/10.1016/j.ccell.2019.09.001 (Original work published December 2019) [PUBMED]
- 60 Testoni, M., Chung, E., Priebe, V., & Bertoni, F. (2015). The transcription factor ETS1 in lymphomas: friend or foe? Leukemia & Lymphoma, 56(7), 1975–80. http://doi.org/10.3109/10428194.2014.981670 (Original work published July 2015) [PUBMED]
- 61 Priebe, V., Sartori, G., Napoli, S., Chung, E., Cascione, L., Kwee, I., … Bertoni, F. (2020). Role of ETS1 in the Transcriptional Network of Diffuse Large B Cell Lymphoma of the Activated B Cell-Like Type. Cancers, 12(7). http://doi.org/10.3390/cancers12071912 (Original work published July 2020) [PUBMED]
- 62 Grünewald, T., Cidre-Aranaz, F., Surdez, D., Tomazou, E., de Álava, E., Kovar, H., … Dirksen, U. (2018). Ewing sarcoma. Nature Reviews. Disease Primers, 4(1), 5. http://doi.org/10.1038/s41572-018-0003-x (Original work published December 2018) [PUBMED]
- 63 Gargallo, P., Juan, A., Yáñez, Y., Dolz, S., Segura, V., Castel, V., & Cañete, A. (2020). Precision medicine in Ewing sarcoma: a translational point of view. Clinical & Translational Oncology : Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 22(9), 1440–1454. http://doi.org/10.1007/s12094-020-02298-7 (Original work published September 2020) [PUBMED]
- 64 Yen, C.-C. (2018). Next frontiers in systemic therapy for soft tissue sarcoma. Chinese Clinical Oncology, 7(4), 43. http://doi.org/10.21037/cco.2018.08.04 (Original work published August 2018) [PUBMED]
- 65 Sawazaki, H., Tsujita, Y., & Ito, Y. (2019). Malignant fibrous histiocytoma arising from the renal capsule and gene mutation screening: A case report. Urology Case Reports, 27, 101004. http://doi.org/10.1016/j.eucr.2019.101004 (Original work published November 2019) [PUBMED]
- 66 Franceschini, N., Lam, S., Cleton-Jansen, A.-M., & Bovée, J. , V. (2020). What’s new in bone forming tumours of the skeleton? Virchows Archiv : An International Journal of Pathology, 476(1), 147–157. http://doi.org/10.1007/s00428-019-02683-w (Original work published January 2020) [PUBMED]
- 67 Czarnecka, A., Synoradzki, K., Firlej, W., Bartnik, E., Sobczuk, P., Fiedorowicz, M., … Rutkowski, P. (2020). Molecular Biology of Osteosarcoma. Cancers, 12(8). http://doi.org/10.3390/cancers12082130 (Original work published July 2020) [PUBMED]
- 68 Kim, B., Kim, Y., Kim, M.-H., Na, Y., Jung, D., Seok, S., … Kim, H. (2020). Identification of FES as a Novel Radiosensitizing Target in Human Cancers. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research, 26(1), 265–273. http://doi.org/10.1158/1078-0432.CCR-19-0610 (Original work published December 2020) [PUBMED]
- 69 Zhao, Y., Wang, Z., Wang, Q., Sun, L., Li, M., Ren, C., … Lu, Y. (2020). Overexpression of FES might inhibit cell proliferation, migration, and invasion of osteosarcoma cells. Cancer Cell International, 20, 102. http://doi.org/10.1186/s12935-020-01181-3 (Original work published December 2020) [PUBMED]
- 70 Chetty, R. (2020). Gene of the month: GLI-1. Journal of Clinical Pathology, 73(4), 228–230. http://doi.org/10.1136/jclinpath-2020-206431 (Original work published April 2020) [PUBMED]
- 71 Doheny, D., Manore, S., Wong, G., & Lo, H.-W. (2020). Hedgehog Signaling and Truncated GLI1 in Cancer. Cells, 9(9). http://doi.org/10.3390/cells9092114 (Original work published September 2020) [PUBMED]
- 72 Välimäki, N., Schalin-Jäntti, C., Karppinen, A., Paetau, A., Kivipelto, L., Aaltonen, L., & Karhu, A. (2019). Genetic and Epigenetic Characterization of Growth Hormone-Secreting Pituitary Tumors. Molecular Cancer Research : MCR, 17(12), 2432–2443. http://doi.org/10.1158/1541-7786.MCR-19-0434 (Original work published December 2019) [PUBMED]
- 73 Tirosh, A., Jin, D., De Marco, L., Laitman, Y., & Friedman, E. (2020). Activating genomic alterations in the Gs alpha gene (GNAS) in 274 694 tumors. Genes, Chromosomes & Cancer, 59(9), 503–516. http://doi.org/10.1002/gcc.22854 (Original work published September 2020) [PUBMED]
- 74 Jorns, J. (2019). Breast Cancer Biomarkers: Challenges in Routine Estrogen Receptor, Progesterone Receptor, and HER2/neu Evaluation. Archives of Pathology & Laboratory Medicine, 143(12), 1444–1449. http://doi.org/10.5858/arpa.2019-0205-RA (Original work published December 2019) [PUBMED]
- 75 Liu, Y., Easton, J., Shao, Y., Maciaszek, J., Wang, Z., Wilkinson, M., … Mullighan, C. (2017). The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nature Genetics, 49(8), 1211–1218. http://doi.org/10.1038/ng.3909 (Original work published August 2017) [PUBMED]
- 76 Nguyen, A., Okabe, A., Tse, V., & Tirado, C. (2020). T-Cell Acute Lymphoblastic Leukemia: A Cytogenomic Update. Journal of the Association of Genetic Technologists, 46(2), 59–73. (Original work published December 2020) [PUBMED]
- 77abcdef Amplified and Over-expressed Genes in Cancer Table. The Institute of Cancer Research. 2009-2010. [http://www.amplicon.icr.ac.uk/table.php]
- 78 Cerione RA, Zheng Y. "The Dbl family of oncogenes." Curr Opin Cell Biol (1996). 8(2): 216-22. [PUBMED]
- 79 Carlo M. Croce, M.D."Oncogenes and Cancer". N Engl J Med 2008; 358:502-511. [http://www.nejm.org/doi/full/10.1056/NEJMra072367]
- 80 Gorgoulis VG, Zacharatos P, Mariatos G, Liloglou T, Kokotas S, Kastrinakis N, Kotsinas A, Athanasiou A, Foukas P, Zoumpourlis V, Kletsas D, Ikonomopoulos J, Asimacopoulos PJ, Kittas C, Field JK. "Deregulated expression of c-mos in non-small cell lung carcinomas: relationship with p53 status, genomic instability, and tumor kinetics." Cancer Res (2001). 61(2): 538-49. [PUBMED]
- 81abcd Gustave Roussy, Cytogenomics of cancers: From chromosome to sequence, Molecular Oncology, Volume 4, Issue 4, August 2010, Pages 309-322 [PUBMED]
- 82 Saito S, Miyaji-Yamaguchi M, Nagata K."Aberrant intracellular localization of SET-CAN fusion protein, associated with a leukemia, disorganizes nuclear export."Int J Cancer. 2004 Sep 10;111(4):501-7. [http://www.ncbi.nlm.nih.gov/pubmed/15239126] [PUBMED]
- 83 Pene-Dumitrescu T, Smithgall TE."Expression of a Src family kinase in chronic myelogenous leukemia cells induces resistance to imatinib in a kinase-dependent manner."J Biol Chem. 2010 Jul 9;285(28):21446-57. Epub 2010 May 7. [http://www.ncbi.nlm.nih.gov/pubmed/20452982/] [PUBMED]
- 84 O'Neil J, Shank J, Cusson N, Murre C, Kelliher M. "TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB."Cancer Cell. 2004 Jun;5(6):587-96. [http://www.ncbi.nlm.nih.gov/pubmed/15193261] [PUBMED]
- 85 Pancewicz J, Taylor JM, Datta A, Baydoun HH, Waldmann TA, Hermine O, Nicot C."Notch signaling contributes to proliferation and tumor formation of human T-cell leukemia virus type 1-associated adult T-cell leukemia."Proc Natl Acad Sci U S A. 2010 Sep 21;107(38):16619-24. Epub 2010 Sep 7. [http://www.ncbi.nlm.nih.gov/pubmed/20823234] [PUBMED]
- 86 Masuda M, Maruyama T, Ohta T, Ito A, Hayashi T, Tsukasaki K, Kamihira S, Yamaoka S, Hoshino H, Yoshida T, Watanabe T, Stanbridge EJ, Murakami Y. "CADM1 interacts with Tiam1 and promotes invasive phenotype of human T-cell leukemia virus type I-transformed cells and adult T-cell leukemia cells."J Biol Chem. 2010 May 14;285(20):15511-22. Epub 2010 Mar 9. [http://www.ncbi.nlm.nih.gov/pubmed/20215110] [PUBMED]
- 87 Larson Y, Liu J, Stevens PD, Li X, Li J, Evers BM, Gao T. "Tuberous sclerosis complex 2 (TSC2) regulates cell migration and polarity through activation of CDC42 and RAC1." J Biol Chem. 2010 Aug 6;285(32):24987-98. Epub 2010 Jun 8. [http://www.ncbi.nlm.nih.gov/pubmed/20530489] [PUBMED]
- 88 Liraz Harel, Barbara Costa, Mike Fainzilber On the Death Trk Department of Biological Chemistry, Weizmann Institute of Science, 76100 Rehovot, Israel [http://www.ncbi.nlm.nih.gov/pubmed/20186708] [PUBMED]
- 89 Introgen [http://www.introgen.com/infotp.html]
- 90 "Atlas of Genetics and Cytogenetics in Oncology and Haematology." (2002) [http://www.infobiogen.fr/services/chromcancer/Genes/P53ID88.html]
- 91ab Vogelstein B, Kinzler KW. "Achilles' heel of cancer?" Nature (2001). 412(6850): 865-866. [PUBMED]
- 92 Soussi T. "The p53 tumor suppresor gene: from molecular biology to clinical investigation." Annals of the New York Academy of Sciences (June 2000). 910: 121-139. [PUBMED]
- 93 Fighting Cancer with Biotechnology. [http://www.biotechinstitute.org]
- 94 Lane DP, et al. "Regulation of p53 stability. The role pf Mdm2 and nuclear export." CRC Laboratories. University of Dundee. (2002). [http://bst.portlandpress.com/bst/027/027668b06.pdf]
- 95 Li-Fraumeni Syndrome. LFS - Genetic Information. OMIM, National Center for Biotechnology Information. Accessed 10/2/2010 [http://www.ncbi.nlm.nih.gov/omim/151623]
- 96 Senturk S, Yao Z, Camiolo M, Stiles B, Rathod T, Walsh AM, Nemajerova A, Lazzara MJ, Altorki NK, Krainer A, Moll UM, Lowe SW, Cartegni L, Sordella R. p53¿ is a transcriptionally inactive p53 isoform able to reprogram cells toward a metastatic-like state. Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):E3287-96. Epub 2014 Jul 29. [PUBMED]
- 97 Igenetics. Russell PJ. Published by Benjamin Cummings: San Francisco, CA: 2002.
- 98 Polyak K, Xia Y, Zweier J, Zinzler K, Vogelstein B. "A model of p53-induced apoptosis." Nature (1997). 389: 300-305. [PUBMED]
- 99 Dameron KM, Volpert OV, Tainsky MA, Bouck N. "Control of angiogenesis in fibroblasts by p53 regulation of angiogenesis of thrombospondin-1." Science (1994). 265: 1582-1584. [PUBMED]
- 100 Zambetti M. "Mdm-2: 'big brother' of p53." J Cell Biochem (1997). 64(3): 343-352. [PUBMED]
- 101 Ramel S, Sanchez CA, Schimke K, Neshat K, Cross SM, Raskind WH, Reid BJ. "Inactivation of p53 and the development of tetraploidy in the elastase-SV40 T antigen transgenic mouse pancreas." Pancreas (1995). 11: 213-222. [PUBMED]
- 102 Tyner, S., Venkatachalam, S., Choi, J., Jones, S., Ghebranious, N., Igelmann, H., … Donehower, L. (2002). p53 mutant mice that display early ageing-associated phenotypes. Nature, 415(6867), 45–53. (Original work published January 2002) [PUBMED]
- 103 Makino, Y., Hikita, H., Fukumoto, K., Sung, J., Sakano, Y., Murai, K., … Takehara, T. (2022). Constitutive activation of the tumor suppressor p53 in hepatocytes paradoxically promotes non-cell autonomous liver carcinogenesis. Cancer Research. http://doi.org/10.1158/0008-5472.CAN-21-4390 (Original work published June 2022) [PUBMED]
- 104 Geradts J, Ingram CD. "Abnormal expression of cell cycle regulatory proteins in ductal and lobular carcinomas of the breast." Modern Pathology (2000). 13(9): 945-953. [PUBMED]
- 105 Knudson, Alfred G. "Mutation and Cancer: Statistical Study of Retinoblastoma." <i>Proceedings of the National Academy of Sciences of the United States of America.</i> 68 (1971): 820-823. [PUBMED]
- 106 Poznic M. Retinoblastoma protein: a central processing unit. J Biosci. 2009 Jun;34(2):305-12. [PUBMED]
- 107 Herwig S, Struss M. "The retinoblastoma protein: a master regulator of cell cycle, differentiation, and apoptosis." Eur J Biochem (1997). 246: 581-601. [PUBMED]
- 108 Singh P, Chan SW, Hong W. "Retinoblastoma protein is functionally distinct from its homologues in affecting glucocorticoid receptor-mediated transcription and apoptosis." Journal of Biological Chemistry (2001). 276: 13762-13770. [PUBMED]
- 109 Witkiewicz AK, Ertel A, McFalls JM, Valsecchi ME, Schwartz G, Knudsen ES. RB-pathway Disruption is Associated with Improved Response to Neoadjuvant Chemotherapy in Breast Cancer. Clin Cancer Res. 2012 Jul 20. [Epub ahead of print] [PUBMED]
- 110 Zini N, Trimarchi C, Claudio P, Stiegler P, Marinelli F, Maltarello M, La Sala D, De Falco G, Russo G, Ammirati G, Maraldi N, Giordano A, Cinti C. "pRb2/p130 and p107 control cell growth by multiple strategies and in association with different compartments within the nucleus." Journal of Cellular Physiology (2001). 189: 34-44. [PUBMED]
- 111 DePhino R. "Transcriptional repression: the cancer-chromatin connection." Nature (1998). 391(6667): 533-536. [PUBMED]
- 112 Magnaghi-Jaulin L, Groisman R, Naguibneva I, Lorrain S, LeVillain JP, Troalen F, Trouche D, Harel-Bellan A. "Retinoblastoma protein represses transcription by recruiting a histone deacetylase." Nature (1998). 391(6667): 601-605. [PUBMED]
- 113 Iwamoto M, Ahnen DJ, Maltzman F, Maltzman T. "Expression of beta-catenin and Full Length APC Protein in Normal and Neoplastic Colonic Tissues." Carcinogenesis (2000). 21(11): 1935-1940. [PUBMED]
- 114 Fultz KE, Gerner EW. "APC-Dependent Regulation of Ornithine Decarboxylase in Human Colon Tumor Cells." Molecular Carcinogenesis (2002). 34:10-18. [PUBMED]
- 115 Morin PJ, Vogelstein B, Kinzler KW. "Apoptosis and APC in Colorectal Tumorigenesis." Proc. Natl. Acad. Sci. USA (1996). 93: 7950-7954. [PUBMED]
- 116abc Welcsh PL, King MC. "BRCA-1 and BRCA-2 and the Genetics of Breast and Ovarian Cancer." Human Molecular Genetics (2001). 10(7): 705-713. [https://academic.oup.com/hmg/article/10/7/705/558549] [PUBMED]
- 117 Welcsh PL, King MC. "BRCA-1 and BRCA-2 and the Genetics of Breast and Ovarian Cancer." Human Molecular Genetics (2001). 10(7): 705-713. [BRCA-1 and BRCA-2 and the Genetics of Breast and Ovarian Cancer] [PUBMED]
- 118ab Narod SA, Boyd J. "Current Understanding of Epidemiology and Clinical Implications of BRCA-1 and BRCA-2 Mutations for Ovarian Cancer." Current Opinion in Obstetrics and Gynecology (2002). 14: 19-26. [PUBMED]
- 119 Welcsh PL, King MC. "BRCA-1 and BRCA-2 and the Genetics of Breast and Ovarian Cancer." Human Molecular Genetics (2001). 10(7): 705-713. [https://academic.oup.com/hmg/article/10/7/705/558549] [PUBMED]
- 120 Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007 Oct 1;120(Pt 19):3327-35. [http://www.ncbi.nlm.nih.gov/pubmed/17881494] [PUBMED]
- 121 Johan H. van Es,1 Rachel H. Giles, and Hans C. Clevers. The Many Faces of the Tumor Suppressor Gene APC. Experimental Cell Research 264, 126¿134 [http://www.ncbi.nlm.nih.gov/pubmed/11237529] [PUBMED]
- 122 Greenberg RA. Recognition of DNA double strand breaks by the BRCA1 tumor suppressor network. Chromosoma. 2008 Aug;117(4):305-17. Epub 2008 Mar 28. [http://www.ncbi.nlm.nih.gov/pubmed] [PUBMED]
- 123 Hashemi J, Lindstrom MS, Asker C, Platz A, Hansson J, Wiman KG. "A melanoma-predisposing germline CDKN2A mutation with functional significance for both p16 and p14ARF." Cancer Lett (2002). 180(2): 211-21. [PUBMED]
- 124 Pierceall WE, Reale MA, Candia AF, et al. Expression of a homologue of the deleted in colorectal cancer (DCC) gene in the nervous system of developing Xenopus embryos. Dev Biol 1994; 166:654¿665. [http://www.ncbi.nlm.nih.gov/pubmed] [PUBMED]
- 125 Keino-Masu K, Masu M, Hinck L, et al. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell 1996;87:175¿185. [http://www.ncbi.nlm.nih.gov/pubmed] [PUBMED]
- 126 Grady WM. Making the case for DCC and UNC5C as tumor-suppressor genes in the colon. Gastroenterology. 2007 Dec;133(6):2045-9. [http://www.ncbi.nlm.nih.gov/pubmed/18054576] [PUBMED]
- 127 Gallo A, Cuozzo C, Esposito I, Maggiolini M, Bonofiglio D, Vivacqua A, Garramone M, Weiss C, Bohmann D, Musti AM. "Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation." Oncogene (2002). 21(42): 6434-45. [PUBMED]
- 128 Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009 Mar;133(3):413-22. [http://www.ncbi.nlm.nih.gov/pubmed/19260747] [PUBMED]
- 129 de Caestecker MP, Piek E, Roberts AB. "Role of transforming growth factor-beta signaling in cancer." J Natl Cancer Inst (2000). 92(17): 1388-402. [PUBMED]
- 130 Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009 Apr;21(2):166-76. Epub 2009 Feb 23. [http://www.ncbi.nlm.nih.gov/pubmed/19237272] [PUBMED]
- 131 Starker LF, Carling T. Molecular genetics of gastroenteropancreatic neuroendocrine tumors. Curr Opin Oncol. 2009 Jan;21(1):29-33. [http://www.ncbi.nlm.nih.gov/pubmed/19125015] [PUBMED]
- 132 Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001 Mar 10;264(1):42-55. [http://www.ncbi.nlm.nih.gov/pubmed] [PUBMED]
- 133 Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8573-8. [http://www.ncbi.nlm.nih.gov/pubmed] [PUBMED]
- 134 Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 Tumor Suppressor, Merlin, Regulates Epidermal Development through the Establishment of a Junctional Polarity Complex. Dev Cell. 2010 Nov 16;19(5):727-39. [http://www.ncbi.nlm.nih.gov/pubmed] [PUBMED]
- 135 Giono LE, Manfredi JJ. The p53 tumor suppressor participates in multiple cell cycle checkpoints. J Cell Physiol. 2006 Oct;209(1):13-20. [http://www.ncbi.nlm.nih.gov/pubmed/16741928] [PUBMED]
- 136 Backman S, Stambolic V, Mak T. "PTEN function in mammalian cell size regulation." Curr Opin Neurobiol (2002). 12(5): 516. [PUBMED]
- 137 Yamasaki L. Role of the RB tumor suppressor in cancer. Cancer Treat Res. 2003;115:209-39. [http://www.ncbi.nlm.nih.gov/pubmed/12613199] [PUBMED]
- 138 Kaelin WG Jr. "Molecular basis of the VHL hereditary cancer syndrome." Nat Rev Cancer (2002). 2(9):673-82. [PUBMED]
- 139 Bernstein C, Bernstein H, Payne CM, Garewal H. "DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis." Mutat Res (2002). 511(2): 145-78. [PUBMED]
- 140 Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993 Dec 3;75(5):843-54. [PUBMED]
- 141 Bartel DP. MicroRNAs: target recognition and regulatory functions. Hum Mol Genet. 2005 Apr 15;14 Spec No 1:R121-32 [PUBMED]
- 142 Kusenda B, Mraz M, Mayer J, Pospisilova S. MicroRNA biogenesis, functionality and cancer relevance. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006 Nov;150(2):205-15. [PUBMED]
- 143ab Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007 Feb 1;302(1):1-12. Epub 2006 Aug 16. [PUBMED]
- 144ab Boyd SD. Everything you wanted to know about small RNA but were afraid to ask. Lab Invest. 2008 Jun;88(6):569-78. Epub 2008 Apr 21. [PUBMED]
- 145 Nikitina EG, Urazova LN, Stegny VN. MicroRNAs and human cancer. Exp Oncol. 2012;34(1):2-8. [PUBMED]
- 146abc Schoof CR, Botelho EL, Izzotti A, Vasques Ldos R. MicroRNAs in cancer treatment and prognosis. Am J Cancer Res. 2012;2(4):414-33. Epub 2012 Jun 28. [PUBMED]
- 147abc Corsini LR, Bronte G, Terrasi M, Amodeo V, Fanale D, Fiorentino E, Cicero G, Bazan V, Russo A. The role of microRNAs in cancer: diagnostic and prognostic biomarkers and targets of therapies. Expert Opin Ther Targets. 2012 Apr;16 Suppl 2:S103-9. Epub 2012 Mar 23. [PUBMED]
- 148abc Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012 Mar;32(2):326-48. doi: 10.1002/med.20215. Epub 2010 Nov 9. [PUBMED]
- 149 Ross SA, Davis CD. MicroRNA, nutrition, and cancer prevention. Adv Nutr. 2011 Nov;2(6):472-85. Epub 2011 Nov 3. [PUBMED]
- 150ab Liu X, Liu L, Xu Q, Wu P, Zuo X, Ji A. MicroRNA as a novel drug target for cancer therapy. Expert Opin Biol Ther. 2012 May;12(5):573-80. Epub 2012 Mar 20. [PUBMED]
- 151 Neilson JR, Sharp PA. Small RNA regulators of gene expression. Cell. 2008 Sep 19;134(6):899-902. [PUBMED]
- 152 Dang CV. Links between metabolism and cancer. Genes Dev. 2012 May 1;26(9):877-90. [PUBMED]
- 153 Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol. 2012 Jan;24(1):62-7. [PUBMED]
- 154 Gao P, Sun L, He X, Cao Y, Zhang H. MicroRNAs and the Warburg Effect: New Players in an Old Arena. Curr Gene Ther. 2012 Aug 1;12(4):285-91. [PUBMED]
- 155 Yuxia M, Zhennan T, Wei Z. Circulating miR-125b is a novel biomarker for screening non-small-cell lung cancer and predicts poor prognosis. J Cancer Res Clin Oncol. 2012 Jul 18. [Epub ahead of print] [PUBMED]
- 156 Wang H, Tan G, Dong L, Cheng L, Li K, Wang Z, Luo H. Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS One. 2012;7(4):e34210. Epub 2012 Apr 16. [PUBMED]
- 157 Cho WC. MicroRNAs as therapeutic targets and their potential applications in cancer therapy. Expert Opin Ther Targets. 2012 Aug;16(8):747-59. Epub 2012 Jun 13. [PUBMED]
- 158 Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, Jacobsen BM, Sartorius CA, Richer JK. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene. 2012 Jul 2. doi: 10.1038/onc.2012.275. [Epub ahead of print] [PUBMED]
- 159 Schreiber R, Mezencev R, Matyunina LV, McDonald JF. "Evidence for the role of microRNA 374b in acquired cisplatin resistance in pancreatic cancer cells." Cancer Gene Ther. 2016 May 27. [PUBMED]

