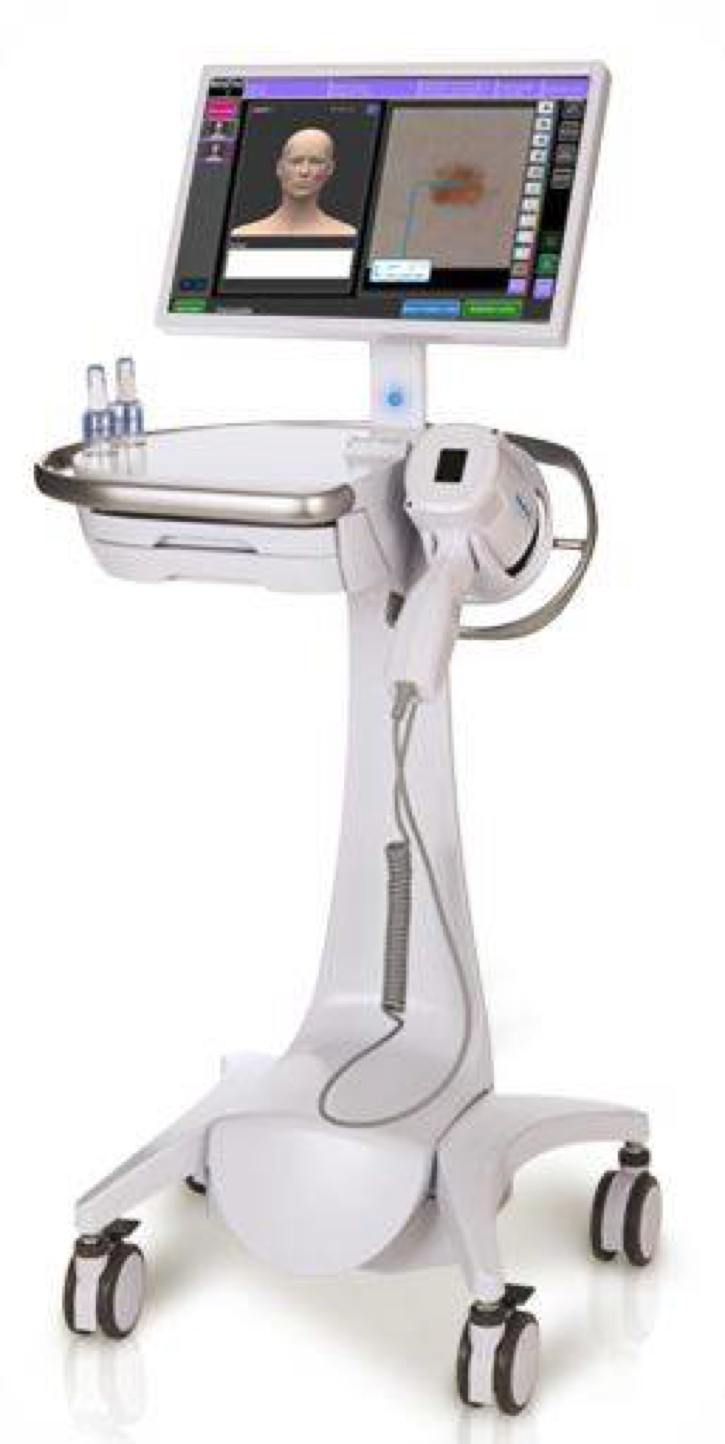
The MelaFind® melanoma detection system was developed by Mela Sciences a clinical dermatology company.1 The system is used to help evaluate suspicious skin abnormalities (lesions). Specifically, the test is used to determine whether a particular colored (pigmented) skin lesion is likely to be melanoma. MelaFind® uses a sophisticated imaging system to capture photographs of the skin abnormality. The photograph is then compared to a database created from 10,000 skin biopsies. The result is a simple 'yes' or 'no'. The result can be used to help guide biopsy decisions. In clinical trials, MelaFind® was found to be very sensitive in detecting melanoma.2 The test was approved by the FDA in November 2011.3
MelaFind® is not used on all skin lesions or all part of the body. According to the manufacturer's website, MelaFind® is effective at finding melanomas ranging in size from 0.2 cm to 2.2 cm (2.54 cm = 1 inch). Larger or smaller lesions are not readily imaged by the system's cameras.
- 1 Mela Sciences Website [http://www.melasciences.com]
- 2 Monheit G, Cognetta AB, Ferris L, Rabinovitz H, Gross K, Martini M, Grichnik JM, Mihm M, Prieto VG, Googe P, King R, Toledano A, Kabelev N, Wojton M, Gutkowicz-Krusin D. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011 Feb;147(2):188-94. Epub 2010 Oct 18. [http://archderm.ama-assn.org/cgi/content/full/147/2/188] [PUBMED]
- 3 FDA New Device Approvals and Clearances. MelaFind® - P090012 [http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm280864.htm]
