The influence of the tissues surrounding a tumor has been recognized for many years. In 1976, to examine the effect of the environment on tumor growth, rats were treated with a carcinogen to cause mutations. They were then given a drug to inhibit the growth of normal liver cells and part of the liver was removed to provide a strong growth stimulus. Under these conditions, the only cells able to grow were those with mutations that allowed them to avoid the growth inhibitory effect of the drug (i.e. cancer cells). In rats given the carcinogen but not the growth inhibitor, no tumors developed. This experiment suggested that tumors cannot grow when the surrounding tissue is normal; in other words, growth of a tumor from a single mutated cell can only occur when the stromal environment is altered in such a way to allow unrestrained tumor growth.1
Further information on the topics on this page can also be found in most introductory Biology textbooks, a good one is Campbell Biology, 11th edition.2
Below is a list of the information found within this section:
- Conditions Inside a Tumor
- Inflammatory Cells and Cancer
- Fibroblasts and Cancer
- Exosomes and Cancer
- Matrix Metalloproteinases and Cancer
- Summary: Tumor-Host Interactions
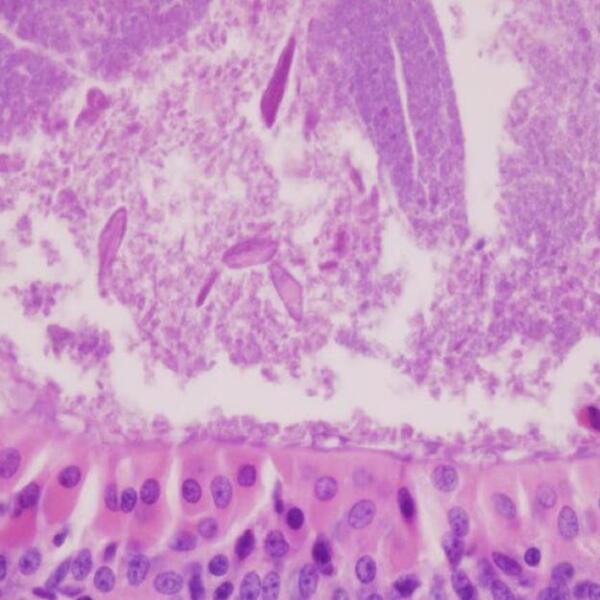
Conditions Inside a Tumor
Tumors are complex structures containing many different kinds of cells. For many years scientists focused their research on understanding the transformation of normal cells to into neoplastic, or cancer, cells but spent little time studying other cells present within a tumor. However, within the past several years, it has become evident that other components of tumors, including resident non-cancerous cells (fibroblasts, endothelial cells), connective tissue, and extracellular matrix (ECM; components of tissues that provide structural support, such as proteins like collagen) are equally important both in tumor initiation (early development) and progression. Collectively, these components are known as the stroma. Many researchers prefer a broader term, the tumor microenvironment, instead of the stroma, as it encompasses infiltrating cells of the immune system (macrophages, lymphocytes) and cell free molecules (growth factors, proteases), in addition to the more or less permanent stromal components 3 As the role of the tumor environment in cancer has become better understood, researchers are hopeful that novel therapeutic agents can be developed that target not just cancer cells, but the environment around them. Drugs targeting both cancer cells and stromal components may be significantly more effective than those directed solely against cancer cells.
The components of the tumor microenvironment can be grouped into four categories: (1) cancer cells, (2) non-cancer cells, (3) secreted soluble factors, (4) and non-cellular solid material such as the ECM 4. The actual composition of the tumor microenvironment is highly variable, with differences seen between patients and often in different areas of the same tumor. The tumor microenvironment is often altered as the disease progresses; even the percentage of a tumor made up of cancer cells may change 5
Communication between a tumor and its surroundings is very important. Both pro- and anti-tumor interactions occur that act to enhance or block tumor formation/progression. For example, one critical molecule, transforming growth factor beta (TGF-Β), is a critical regulator of tumor progression. TGF-Β is a potent inhibitor of cell growth and is secreted by multiple cell types within the tumor microenvironment; however, mutations in many advanced carcinomas result in cancer cells that are unaffected by TGF-Β (meaning they continue to grow even in the presence of TGF-Β). In addition, tumors themselves often secrete TGF-Β, which reduces the growth of surrounding normal cells, thus allowing the tumor cells to reproduce rapidly without competition from neighboring cells 3 In this way, tumors continue to grow at the expense of surrounding cells.
The conditions within the tumor microenvironment differ considerably from those in normal tissue. Major changes include:
- Hypoxia (low oxygen levels)
- Low pH (acidic conditions)
- Low glucose levels
In addition, massive cell death occurs, resulting in the release of proteins and other molecules into the surrounding environment. These factors may help or hurt tumor growth.3 Hypoxia results in the generation of oxygen free radicals which lead to DNA damage (mutation). Mechanisms for repairing this damage are also less efficient under hypoxic conditions. The end result is an increase in the mutation rate and greater variation within the tumor population. Another result is that only those cells with mutations that allow them to survive in harsh conditions will continue to grow and contribute to the tumor 6
Importantly, the conditions within the tumor microenvironment affect more than just cancer cells. The normal cells surrounding a tumor exhibit altered characteristics compared to corresponding cells in normal tissue. These cells also develop mutations, and the tissue is often disorganized compared to normal tissue 5 These abnormal properties might arise in two ways. The conditions of the tumor microenvironment (hypoxia and low pH) may induce mutations, or soluble products (growth factors, cytokines) released from the tumor may alter the genes expressed by stromal cells 3 Interestingly, mutations have been identified in the stroma of non-cancerous tissue collected from breast cancer patients, suggesting that pre-existing genetic alterations in the stroma may provide the foundation for tumor initiation 6Experiments have illustrated the importance of the stroma in tumor development.
Inflammatory Cells and Cancer
The role of the immune system in cancer is a double-edged sword. While there is evidence that a strong immune system can be beneficial, in many cases the immune system clearly promotes tumor growth7. For example, patients with weak immune systems (immunosuppressed), have a higher incidence of cancer, but on the other hand, innate immune cells are believed to contribute to tumor formation through the release of molecules that regulate cell growth and migration, and angiogenesis 8. Innate immune cells, such as macrophages that do not produce antibodies but are capable of ingesting foreign organisms, are prominent in pre-malignant and malignant tissues. In addition, many cancers (gastric, cervical, colon, liver) are associated with infection and correlate with the activity of the normal host immune response. Chronic inflammatory conditions make people more likely to develop certain cancers; for example, patients with Crohn's disease have a higher incidence of colorectal cancer. A greater understanding of the ways by which the inflammatory response initiates cancer may lead to potent new cancer treatments 7.
In other cases, the tumor itself attracts immune cells which can then impact tumor progression. Tumor cell damage and hypoxia attract macrophages from the blood into the tissue surrounding a tumor. In most cases, high tumor associated macrophage (TAM) counts are correlated with reduced survival. Many tumors secrete factors that prevent macrophages from alerting other immune cells to the presence of cancer cells, resulting in an inability of the immune system to recognize the tumor. Macrophages themselves secrete factors that enhance tumor cell proliferation, invasion, and promote angiogenesis. In addition, TAMs release oxygen free radicals and other mutagenic compounds that may create mutations in surrounding cells. The ability of TAMs to stick to tumor cells allows macrophages to carry tumor cells into the circulation and thus aid in the spread of the cancer (metastasis) 9, 6, 8
Learn more about inflammation and cancer.
Learn more about the immune system.
Fibroblasts and Cancer
Fibroblasts are the predominant cells in the stroma. They are responsible for generating the extracellular matrix (ECM) as well as connective tissue. Because each tissue has different requirements, fibroblasts from different organs express different genes. Changes in fibroblast behaviors are associated with tumor progression, mostly due to factors made by the tumor. Fibroblasts begin to express α-SMA (alpha-smooth muscle actin), which allows them to contract. These myofibroblasts are highly proliferative and are surrounded by a dense meshwork of the structural protein collagen. This profile is known as desmoplasia and is often associated with recruitment of immune cells and angiogenesis 10, 8.
Interestingly, although the behavior (phenotype) of fibroblasts is often altered by close proximity to a tumor, in other cases altered fibroblasts have been isolated from patients with no cancer, but who have hereditary predispositions to the disease. This observation suggests that these altered fibroblasts may actually aid in the development of cancer11.
How might these cells become oncogenic in the absence of a tumor? Several possibilities exist, including exposure to carcinogens, accumulation of genetic damage due to aging, and hormone imbalances. Molecules present in healing wounds can also alter fibroblasts in such a way that they resemble fibroblasts found near tumors.8
Exosomes
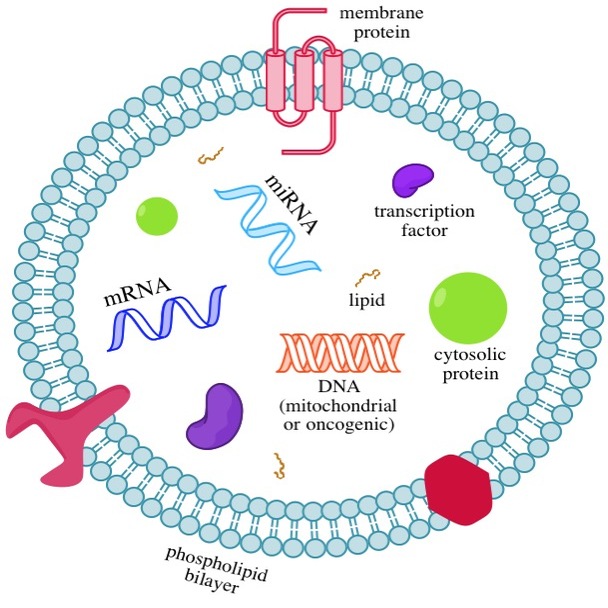
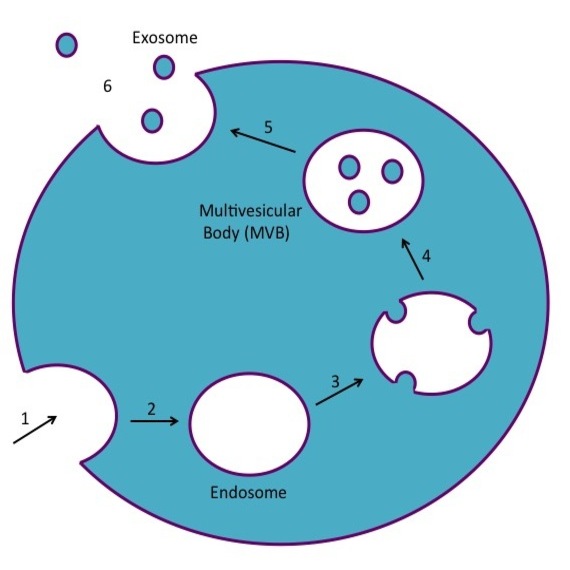
Exosomes released from cancer cells can change the structure of tissues to make it easier for cancer to spread (metastasize).
Matrix Metalloproteinases and Cancer
One of the most critical roles performed by fibroblasts, both in normal and cancer tissue, is the production and remodeling of the extracellular matrix (ECM). Not only does the ECM impart structural support and strength to tissues, it also provides attachment sites for cell surface receptors, and functions as a reservoir of cytokines and other growth factors24 The structure of tumor-associated ECM is abnormal, with loose structure and disorganized collagen fibers25 Matrix metalloproteinases (MMPs) are a large family of enzymes capable of degrading components of the ECM and are critical in maintenance of the ECM. Degradation of the ECM by MMPs releases growth factors, enhances migration, and alters cell:cell and cell:ECM interactions26. Although MMPs can be produced by tumor cells, most are produced by fibroblasts and macrophages, and high levels of MMPs are found at the tumor:stroma interface5. Because MMPs are secreted into the surrounding environment by these cells, they are a good example of the interaction that occurs between a tumor and its environment.
Evidence indicates that MMPs are key players in multiple steps of tumor progression; they promote metastasis, angiogenesis, and even tumor initiation. One of the many paradoxes of MMP activity is that MMPs often have opposing effects depending on the composition of the tumor environment and the nature of MMPs present. For example, MMPs can either promote or inhibit angiogenesis, depending on the molecules they release from the ECM27, 26. Because of their potent effects on tumor formation and metastasis, several clinical trials attempted to use MMP inhibitors as anticancer therapy. However, these trials were soon stopped as patients developed muscle and bone pain, formed connective tissue nodules, and developed joint disorders. These trials highlight the difficulty of targeting molecules critical for the function of multiple tissues27.
Summary: Tumor-Host Interactions
Tumor Microenvironment
- The tumor microenvironment consists of four components:
- Cancer cells
- Non-cancer cells
- Secreted soluble factors
- Non-cellular, solid material
- The actual composition of the tumor microenvironment is highly variable.
Conditions within the tumor microenvironment
- Low oxygen levels (hypoxia), acidic conditions (low pH), and low sugar (glucose) levels are common conditions in tumors.
- Conditions within the tumor microenvironment affect both cancer cells and normal cells.
- The tissue within and surrounding a tumor is often disorganized.
Inflammatory Cells in Cancer
- The immune system can inhibit or promote tumor growth.
- Many cancers are associated with chronic inflammatory conditions that activate cells of the innate immune system.
- Macrophages secrete factors that enhance tumor cell proliferation, invasion, and promote angiogenesis.
Fibroblasts in Cancer
- Fibroblasts are the predominant cells in the stroma.
- Changes in fibroblast behavior are associated with tumor progression.
- Matrix metalloproteinases (MMPs) produced by fibroblasts degrade the extracellular matrix.
- MMPs are key players in cancer initiation, metastasis, and angiogenesis.
Exosomes and Cancer
- Exosomes are small membrane-covered vessels made by cancer cells and other cells
- Exosomes carry proteins and nucleic acids
- When they travel to distant parts of the body, exosomes can alter the immune response and/or enhance the spread of cancer
Matrix Metalloproteases and the Tumor Microenvironment
The Tumor Stroma and Metastasis
- Seed and Soil hypothesis: given tumor cells (seeds) can only colonize particular distant tissues (soil) that have a suitable growth environment.
- Two key events must occur for site-specific metastasis to occur: 1) formation of a viable landing spot and 2) expression of appropriate genes in the tumor cells.
- Tumor cells may invade foreign tissue but fail to colonize it. The reasons for this are unknown. These cells are considered 'dormant' cancer cells.
- 1 Solt D., Farber E. A new principle for the analysis of chemical carcinogenesis. Nature. 263:701-703. 1976.
- 2 Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., & Reece, J. B. (2017). Campbell Biology (11th ed.). Pearson.
- 3abcd Witz, I.P., and O. Levy-Nissenbaum. 2006. The tumor microenvironment in the post-PAGET era. Cancer Lett. 242:1-10. [PUBMED]
- 4 Cretu, A., and P.C. Brooks. 2007. Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imaging opportunities. J Cell Physiol. 213:391-402. [PUBMED]
- 5abc Zalatnai, A. 2006. Molecular aspects of stromal-parenchymal interactions in malignant neoplasms. Curr Mol Med. 6:685-93. [PUBMED]
- 6abc Laconi, E. 2007. The evolving concept of tumor microenvironments. Bioessays. 29:738-44. [PUBMED]
- 7ab Kopfstein, L., and G. Christofori. 2006. Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci. 63:449-68. [PUBMED]
- 8abcd Tlsty, T.D., and L.M. Coussens. 2006. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 1:119-50. [PUBMED]
- 9 Condeelis, J., and J.W. Pollard. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 124:263-6. [PUBMED]
- 10 Beacham, D.A., and E. Cukierman. 2005. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 15:329-41. [PUBMED]
- 11 Schor, S.L., J.A. Haggie, P. Durning, A. Howell, L. Smith, R.A. Sellwood, and D. Crowther. 1986. Occurrence of a fetal fibroblast phenotype in familial breast cancer. Int J Cancer. 37:831-6. [PUBMED]
- 12ab Zhang, Xuan, Zenglin Pei, Jinyun Chen, Chunxia Ji, Jianqing Xu, Xiaoyan Zhang, and Jin Wang. "Exosomes for Immunoregulation and Therapeutic Intervention in Cancer." J. Cancer Journal of Cancer 7.9 (2016): 1081-087. [http://www.ncbi.nlm.nih.gov/pubmed/27326251] [PUBMED]
- 13abcde Zhou, Jianbiao, Sam Wang, Kangyun Sun, and Wee-Joo Chng. "The Emerging Roles of Exosomes in Leukemogeneis." Oncotarget (2015) [http://www.ncbi.nlm.nih.gov/pubmed/27191983] [PUBMED]
- 14abcde Isola, Allison L., and Suzie Chen. ¿Exosomes: The Link between GPCR Activation and Metastatic Potential?¿ Frontiers in Genetics 7 (2016): 56. PMC. [http://www.ncbi.nlm.nih.gov/pubmed/27092178] [PUBMED]
- 15 Raposo, Graça, and Willem Stoorvogel. "Extracellular Vesicles: Exosomes, Microvesicles, and Friends." J Cell Biol The Journal of Cell Biology 200.4 (2013): 373-83. [http://www.ncbi.nlm.nih.gov/pubmed/23420871] [PUBMED]
- 16 Zhang, Jian et al. ¿Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function.¿ Genomics, Proteomics & Bioinformatics 13.1 (2015): 17¿24. PMC. [http://www.ncbi.nlm.nih.gov/pubmed/25724326] [PUBMED]
- 17abcd Tickner, Jacob A. et al. ¿Functions and Therapeutic Roles of Exosomes in Cancer.¿ Frontiers in Oncology 4 (2014): 127. PMC. [http://www.ncbi.nlm.nih.gov/pubmed/24904836] [PUBMED]
- 18 Bockhorn, Maximilian, Rakesh K Jain, and Lance L Munn. ¿Active versus Passive Mechanisms in Metastasis: Do Cancer Cells Crawl into Vessels, or Are They Pushed?¿ The lancet oncology 8.5 (2007): 444¿448. PMC. [http://www.ncbi.nlm.nih.gov/pubmed/17466902] [PUBMED]
- 19 Peinado, Héctor et al. ¿Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a pro-Metastatic Phenotype through MET.¿ Nature medicine 18.6 (2012): 883¿891. PMC. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3645291/] [PUBMED]
- 20ab Tran, Thanh-Huyen, George Mattheolabakis, Hibah Aldawsari, and Mansoor Amiji. "Exosomes as Nanocarriers for Immunotherapy of Cancer and Inflammatory Diseases." Clinical Immunology 160.1 (2015): 46-58. [http://www.ncbi.nlm.nih.gov/pubmed/25842185] [PUBMED]
- 21 Johnsen, Kasper Bendix, Johann Mar Gudbergsson, Martin Najbjerg Skov, Linda Pilgaard, Torben Moos, and Meg Duroux. "A Comprehensive Overview of Exosomes as Drug Delivery Vehicles ¿ Endogenous Nanocarriers for Targeted Cancer Therapy." Biochimica Et Biophysica Acta (BBA) - Reviews on Cancer 1846.1 (2014): 75-87. [http://www.ncbi.nlm.nih.gov/pubmed/24747178] [PUBMED]
- 22 Romagnoli, Graziela Gorete et al. ¿Dendritic Cell-Derived Exosomes May Be a Tool for Cancer Immunotherapy by Converting Tumor Cells into Immunogenic Targets.¿ Frontiers in Immunology 5 (2014): 692. PMC. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4298225/]
- 23 Mahaweni, Niken M. et al. ¿Tumour-Derived Exosomes as Antigen Delivery Carriers in Dendritic Cell-Based Immunotherapy for Malignant Mesothelioma.¿ Journal of Extracellular Vesicles 2 (2013): 10.3402/jev.v2i0.22492. PMC. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3823268/]
- 24 Badylak, S.F. 2002. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 13:377-83. [PUBMED]
- 25 Sivridis, E., A. Giatromanolaki, and M.I. Koukourakis. 2004. "Stromatogenesis" and tumor progression. Int J Surg Pathol. 12:1-9. [PUBMED]
- 26ab Jodele, S., L. Blavier, J.M. Yoon, and Y.A. DeClerck. 2006. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 25:35-43. [PUBMED]
- 27ab Duffy, M.J., P.M. McGowan, and W.M. Gallagher. 2008. Cancer invasion and metastasis: changing views. J Pathol. 214:283-93. [PUBMED]
