The immune system consists of a large number of different types of cells and proteins that function to distinguish between normal and abnormal cellular components and between 'self' and 'non-self'. As an example, when a thorn gets stuck in the body, the immune cells are able to recognize the thorn as a foreign object (i.e. 'non-self') and attack it. The same is true for bacteria, viruses or other organisms that can invade our bodies. A more subtle distinction between self and non-self occurs in the recognition of cancer cells by the forces of the immune system. The cancer cells are recognized and attacked because they differ from the normal 'self' from which they arose.
The cells and proteins of the immune system participate in two broad and somewhat overlapping types of immunity-Non-specific and Specific1 Further information on the topics on this page can also be found in most introductory Biology textbooks, we recommend Campbell Biology, 11th edition.2
The sections that follow describe some major components and activities of the immune system:
- The Innate Immune System
- The Acquired Immune Response
- The Lymphatic System
- The Immune System and Cancer
- Inflammation and Cancer
- Immune System and Inflammation Summary
You might also want to view our section on Cancer Vaccines
The Innate Immune System
The innate immune system gets its name from the fact that we are all born with it already in place, and it changes little throughout our lives. This division of the immune system provides protection by recognizing general features of possible pathogens. For instance, barriers such as the skin block entry of many kinds of organisms. Similarly, cells of the innate immune response recognize general features of pathogens, such as the cell walls of bacteria. These cells do not distinguish within the various classes of pathogens. To use a military analogy, it would be like using the same type of missile to shoot at many different kinds of targets instead of having different missiles for different types of target. Macrophages, for instance, are cells that participate in the innate immune response by finding, eating, and killing many different types of bacteria. Natural killer cells (NK cells) are another type of immune cell that functions to eliminate cells that have become infected with viruses and cancer cells.1
There are several different components of the innate immune system. While is it sometimes called 'non-specific' immunity, that is not really accurate. The defenses presented below are geared toward specific kinds of living and non-living 'invaders'. When an organism or particle attempts to enter the body, there are several physical and chemical barriers that must be bypassed. Our skin is a tough flexible shield that blocks many types of invaders. The entry points to our body are protected by sticky mucus (i.e. mouth, nose, anus, vagina) or wax (ears) that traps bacteria, dust and other particles. Body secretions like the acid in our stomachs and proteins in saliva and tears also work to prevent entry. Our hair keeps larger organisms from reaching our skin. If an invading organism or particle (i.e. a thorn) do make it past these defenses, cells (produced in the bone marrow) like macrophages and neutrophils are waiting to attack the foreign object.
View the graphic below to see some components of the innate immune system in humans.

The Acquired Immune Response
Specific or adaptive immunity is the second line of defense because it is initiated if the non-specific, innate immune response is unable to completely combat the invading pathogen. The two systems really overlap somewhat. As an example, proteins produced by cells of the adaptive immune system are present in secretions like tears that also contain proteins that are part of the innate immune system.
The adaptive response develops and changes over the course of our lifetimes and is thus also called acquired immunity. The cells and proteins of the adaptive immune response are highly specific for invading pathogens or abnormal cells within the body. This is in contrast to the broad spectrum activities of the components of the innate immune system.
Like our innate immune system, the specific immune response is composed of several different types of cells and the proteins that they produce. The main cells of the adaptive immune response originate in our bone marrow and mature at different locations in the body. The cells may float around in the blood stream or lymphatic system or take up residence in an organ or tissue. Two of the main cell types spend a significant amount of time in the lymphatic system and are known as lymphocytes. These two types of immune cells are called T cells and B cells. A major protein component of the acquired immune system is the antibodies produced by B cells.1
The specific immune response is an active system with four defining characteristics:
- Antigen Specificity-The cells and proteins of this system only recognize very particular protein fragments (peptides) on other cells or dissolved in body fluids
- Diversity in the number of peptides that can be recognized. The acquired immune system is capable of responding to an astounding number of different foreign proteins. The number of different proteins and organisms we encounter in our lifetime is enormous and the acquired immune system is able to generate a specific response against each one!
- Memory-A hallmark of the acquired immune response is that if the same foreign object is encountered again, the response is both more rapid and more intense. The system remembers the things it has encountered. This is accomplished by the generation of 'memory' cells that live for a long time, waiting for their chance to re-activate and lead the charge.
- Self:Non-Self Discrimination-The acquired immune system is able to recognize cells that have been altered in very minor ways and respond appropriately. An example of self:non-self discrimination is the rejection of an organ following transplantation. A kidney from one person may be recognized as 'non-self' by the recipient and destroyed. For this reason, transplant patients receive medications that lower their immune response.1
Of importance to us: The genetic changes that make normal cells into cancer cells can also alter them in ways that can be detected by the immune system.
Cells of the Acquired Immune System
The main cells of the specific immune response are lymphocytes - B cells and T cells. All lymphocyte precursors originate in the bone marrow. The pre-B cells stay in the bone marrow to undergo further development, while the T cell precursors migrate to an immune organ located in the neck (the thymus) to further develop. In fact, T cells get their name from the thymus. For trivia buffs: B cells are named after an organ found in chickens (the bursa of Fabricius) where they were first studied. Humans do not have an equivalent organ.
Early in T cell and B cell development, developing cells that strongly react with normal cell proteins are removed from the system. In this way, the immune system ensures that the B cells and T cells do not kill normal body cells. If self-reactive T cells and B cells are not removed from the lymphocyte population, autoimmune diseases like lupus or rheumatoid arthritis may develop.
There are two classes of mature T cells:
- Helper T cells- These cells help other immune cells, including CTLs, macrophages and B cells, carry out their functions more efficiently.
- Cytotoxic T Lymphocytes (CTL)-(cyto=cell and toxic because they can kill) These are cells that are able to kill other cells, they are cellular assasins. They directly kill any cell that they recognize as abnormal, such as cells infected with viruses or cancer cells.
The immature T cells residing in the lymph nodes and spleen do not mature into full effector cells until an APC comes to them and shows them, or presents to them, a particular protein antigen. Once the T cell is notified by the APC that there are cells in the body expressing these abnormal proteins, the T cells mature and leave the lymph nodes and the spleen to circulate in the body and find the abnormal cells. When the T cells find the abnormal cells they are able to kill them. In the case of virus infection, killing the infected cell is a harsh but effective way to limit the production of the viruses within. Cancer cells may also be recognized and eliminated by cytotoxic cells of the immune system.
B cells are another critical component of the acquired immune response. Like T cells, B cells are formed in the bone marrow. The cells move out into the body to mature. B cells are responsible for producing antibodies, proteins that recognize foreign objects that enter the body (viruses, bacteria, other proteins, etc.). Different B cells can recognize different targets. There are millions of different kinds of B cells in our bodies and our immune system can respond to a very large number of different 'foreign' targets.
The immune system functions as an effective surveillance system to eliminate abnormal cells and invading organisms from our bodies.
How the Immune System Sees the World
Our immune system constantly surveys our body checking for invaders, like bacteria and viruses. The system is also able to recognize when normal cells become altered such as cancer cells. Recognition of invaders or altered 'self' involves cooperation between different cells and is tightly controlled.
The exact steps involved in the generation of an immune response are slightly different depending on the type of threat (virus, bacteria, etc.) but in general, our cells recognize small parts of the target, usually protein fragments that are created by the digestion of a larger protein. For example, a bacterium that invades the skin via a wound may be recognized by the proteins on its surface.
A protein or other product (sugar, lipid, etc.) that can be recognized by the immune system and lead to the production of an immune response is known as an antigen.
Some immune cells, including macrophages and dendritic cells, are able to carry these proteins on their surface, like waving a flag! The fragments of proteins (antigens) are 'presented' to the B and T cells and cause those cells to become active. The cells that are able to present antigens are known, understandably enough, as Antigen Presenting Cells or APC.
Individual B cells and T cells each express a single type of receptor molecule on their cell membrane. They do have many copies of that receptor on their surface. These receptors are called B-cell receptors (BCR, or immunoglobulins) and T-cell receptors (TCR). Each of these receptors binds to just one very specific peptide (antigen) from an abnormal cell or foreign object. The expression of a single type of receptor ensures that each lymphocyte is specific for just one antigen. Unlike the cells of the innate immune response, lymphocytes can distinguish between very similar target molecules. There are enought different lymphocytes in the body to recognize more than one billion different peptides! This amazing diversity assures that there are cells that are able to recognize just about any target encountered in our lifetimes.
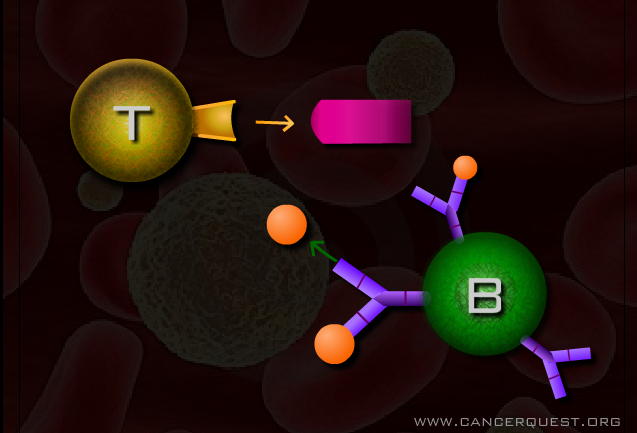
The specific immune response is divided into two parts, humoral and cellular immunity. Humoral immunity is dependent on the production of specific proteins known as antibodies. Antibodies are produced by B cells. These protein interceptors are are small Y-shaped molecules that circulate in blood and other body fluids. When an antibody bumps into its specific target (antigen) it binds tightly allowing the target to be destroyed or inactivated. Antibodies can
- Neutralize toxins
- Bind to viruses to prevent their entry into cells
- Bind molecules in the bloodstream leading to their clearance
- Mark the target for consumption by of the non-specific immune system
- Work with other proteins in the body to directly kill bacteria and parasites
The Lymphatic System
Once they are formed in the bone marrow, lymphocytes circulate in the body and reside in lymphatic tissue, including lymph nodes and the spleen, where they search for and await contact with their target proteins. The lymphatic system is a system of vessels (tubes) all throughout the body. Like the more familiar circulatory system, the lymphatic system carries fluid, proteins and cells of the immune system. Red blood cells are not found in the lymphatic system. The two systems (lymphatic and circulatory) are connected. The lymphatic system picks up fluid and cells from around the body and returns them to the circulatory system via ducts located in the neck/shoulder area. The fluid within the vessels is known as lymph.
Like smaller streams merging into rivers that ultimately flow into an ocean, small lymphatic vessels empty their contents into larger ones. The flow leads to collections of grape-like structures knowns as lymph nodes. Many cells in the immune system reside in the lymphatic system for much of their existence.

The lymphatic system is of great importance in cancer for several reasons:
- Cancer cells can spread (metastasize) by getting into the lymphatic system.
- Many cancer types are classified or staged by whether or not cancer cells can be found in lymph nodes close to the site of the original tumor. The logic is this: The lymphatic system is found all over the body so if cancer cells from a tumor have made it that far, they may also have traveled to distant locations.
Learn more about the lymphatic system and metastasis.
The Immune System and Cancer
It was not always clear to scientists that the immune system played a role in preventing and combating cancer. This idea was proposed in 1957, but the scientific evidence at the time only seemed to indicate that the immune system protected against pathogens like viruses and bacteria, but not against abnormal body cells like cancer cells. Researchers and doctors in the late 1900s noticed, however, that people with extremely weak or no immune system had a greater risk of developing cancer than the average person. In addition, researchers have since noticed that patients with immune cells present in their tumors have a better prognosis than patients without immune cells in their tumors. 3
Immunosurveillance is a term used to describe the action of the immune cells, including T cells, as they move through the body and look for any abnormalities. When cells become mutated, they may appear to the immune cells as abnormal. The body then recognizes them as non-self or foreign. By eliminating cells that have become abnormal, the immune system helps to protect against cancer. However, if the cells mutate enough so that they are able to escape the surveillance mechanisms of the immune system, they may continue to reproduce as cancer cells. The process is a complex version of 'hide and seek' with major consequences.
As described in the previous pages, T cells recognize peptide antigens 'presented' on their cell surface. If pre-cancerous cells present abnormal proteins T cells will recognize these cells as abnormal. Conversely, pre-cancerous cells that the immune system does not recognize as abnormal, or is unable to kill, will survive and may proliferate to form a tumor.
There are many ways that tumor cells may use to get around the immune defenses of the body. Many cancers produce chemical messengers that inhibit the actions of immune cells. Other cancers have defects in the way that antigens are presented on their cell surface. Other immune cells, called natural killer (NK) cells, play a special role in this case, however, because they notice when body cells no longer have present specific 'self' proteins on their surface and kill the abnormal cells. Additionally, some tumors grow in locations such as the eyes or brain, which are not regularly patrolled by immune cells. 4
The main goal of immunotherapy and cancer vaccines is to provide the immune system with the signals that it needs to recognize the cancer cells as abnormal. If successful, these strategies may allow the body to recognize and destroy cancer cells, even those that have been able to form a tumor.
Learn more about cancer vaccines.
The immune system and the development of cancer.
In addition to fighting cancer, the immune system appears to be actively involved in the development of most, if not all, cancers. A unifying feature is long term inflammation. Inflammation is what happens when immune cells secrete chemicals and proteins in response to a 'threat'. The threat can be an invading microbe (bacteria/virus) or is can be much more subtle. We now know that obesity and stress can both trigger an inflammatory response from the immune system. The inflammation can last for a long time, often for many years. It is the long term activity that causes problems for normal cells, and can lead to the development of cancer.
The inflammation seen in cancer is a good response that has gone bad. There are several different kinds of immune cells that are involved, some of which are discussed below. The inflammation seen in cancer is actively being studied as a possible cancer prevention and cancer treatment target.
Macrophages and tissue remodeling
Macrophages are white blood cells responsible for destroying microbes and foreign material. As macrophages flood the area around a tumor, they become part of a complex tumor microenvironment. Macrophages surrounding the tumor are referred to as tumor associated macrophages (TAMs), and often play a role in tumor growth instead of tumor destruction. The presence of macrophages leads to inflammation, which promotes proliferation of the cancer cells, blood vessel growth, cancer cell invasion, spread to distant locations (metastasis), and resistance to cancer treatments, including chemotherapy.
Circulating monocytes (precursors to macrophages) also play a role in the tumor microenvironment. Inflammatory monocytes (IMs), which usually attack microbes, can be harmful because they promote inflammation, and produce proteins that stimulate inflammation, including tumor necrosis factor alpha (TNF-alpha) and interleukin 1 beta (IL-1 beta). Resident monocytes (RMs) normally respond to viruses, and are involved in tissue remodeling, angiogenesis and collagen production. IMs in particular are associated with many cancers.
The way a macrophage acts in the tumor microenvironment can vary. Macrophages that are NOT assisting the growth of the tumor are classified as M1, and those macrophages that are producing products that assist the tumor growth are called M2 macrophages. What induces the change from M1-M2 is currently unknown. Typically, macrophages surrounding a newly developing tumor are M1, and are still working normally to attack the tumor. As the tumor progresses, however, more M2 macrophages are present, promoting pro-tumor activities such as angiogenesis and metastasis. TAMs can also promote local immunosuppression, and as a result, prevent other immune cells from attacking the tumor.5, 6
Bone Marrow Derived Suppressor Cells (Myeloid-derived suppressor cells)
Suppressor cells are immune cells that can block the immune system. In a normally functioning system, we need these cells to reduce or stop the activity of the immune system once a threat has been eliminated. They work to suppress T-cell responses and regulate the production of signaling proteins (cytokines) by macrophages. In cancer and other illnesses, these cells are not acting the way they should, and they can block immune responses against cancer cells.7, 8
Inflammation and Cancer
Inflammation is the body’s response to potentially harmful events. It is a protective and necessary process that involves recruiting cells and molecules of the host’s immune system to the site of injury. The recruited cells and molecules, along with resident cells, remove damaged and dead (necrotic) cells and tissue. They work to eliminate the cause of the irritation, whether it is a chemical, foreign object, or an invasive organism. The immune cells begin the process of repairing the cells and tissues in the area. Inflammation is a complex immune response that involves a large number of different cells and signals. It is essential for our survival.9
Signs of Inflammation
Acute inflammation, first described back in the 1st century AD, is an observable physical response with four key signs, described by the Roman, Aulus Cornelius Celsus:
1. rubor (redness)
2. calor (heat)
3. tumor (swelling)
4. dolor (pain) 11
Centuries later, we now know that there is a different manifestation of inflammation; one that does not display these physical symptoms, and which likely contributes to many diseases affecting humans. This form of inflammation is referred to as chronic inflammation. Chronic inflammation plays an important role in the formation of cancer, contributing to at least 15% of all solid tumors.12
How can inflammation be harmful?
Our immune system works to attack and eliminate foreign invaders, but this defense mechanism, if not controlled, can also be harmful. In particular, inflammation can become harmful to an individual when the process is prolonged. 13 In normal wound healing, inflammation and the cellular reproduction needed to repair damage subside after the injury is dealt with, but if the process is sustained for several years, epidemiologic studies have shown that it increases an individual’s risk for cancer. 13As with many aspects of cancer, inflammation’s role in cancer involves a normal biological process gone wrong. Something that should be over quickly occurs for a prolonged time, or at an inappropriate time. A primary way that chronic inflammation causes cancer involves the production of chemicals that can damage cells. It is similar to the accidental damage caused when weapons go astray. These chemicals, called reactive oxygen species (ROS) and nitric oxide species (NOS), are used by cells of the immune system (leukocytes and phagocytes) as a defense against infections.14ROS and NOS cause DNA damage that can kill invading organisms. If they attack our own cells, they can cause permanent DNA changes (mutations). 13 Immune cells can also produce signaling molecules (cytokines), destructive enzymes (proteases), and other mediators of cell killing (including TNF-α and interleukins).13
Chronic vs Acute Inflammation
Chronic Inflammation vs. Acute Inflammation
Acute (short-term) inflammation is different from chronic (long-term) inflammation in several ways. The differences include:
Acute Inflammation
- Fast onset (minutes or hours)
- Cells recruited are mostly neutrophils
- Causes mild and self-limiting damage to host tissue
- Noticeable local and systemic physical symptoms (the four cardinal signs)9.

Chronic Inflammation
- Slower onset (days)
- Usually involves recruitment of macrophages (derived from monocytes) and lymphocytes
- Causes gradual to severe damage to host tissue
- Subtle or non-existent physical signs9
Most importantly for our purposes, chronic inflammation has been linked with increased cancer risk, and acute inflammation has not.

Acute Inflammation
Acute inflammation is the normal immune response to an infection and is not considered a risk factor for cancer13. It is a universal and immediate response triggered by a local injury. The local injury could be bacterial, viral, fungal, or parasitic infection; blunt or penetrating trauma; tissue necrosis; foreign bodies (e.g. a splinter); or hypersensitivity immune reactions (like those to poison ivy). 9
Acute inflammation can be separated into two major components: changes in blood vessels (vascular changes) and cellular events. In a matter of seconds after an injury occurs, a variety of chemical mediators appear in the tissue and lead to these changes. The vascular changes include increased blood flow, increased vascular permeability, and activation of cells lining the blood vessels (endothelial cells) in order to allow for immune cell (leukocyte) adhesion and migration. The cellular events involve recruitment of white blood cells (WBC, or leukocytes) to the site of injury and activation of leukocytes, which allows them to eliminate any invading organisms. 9 Acute inflammation primarily involves a subset of leukocytes called polymorphonuclear leukocytes or PMN. This is a non-specific response—different types of injuries lead to the same response. 15
Chronic Inflammation
Chronic inflammation is a prolonged immune response that often leads to bystander tissue damage. These responses can last for many years. Chronic inflammation is different from acute inflammation, though acute inflammation can develop into chronic inflammation if the injury/infection is long-lasting or there is something preventing the normal healing process. Chronic inflammation can be triggered by persistent infections, hypersensitivity diseases, and long-term exposure to toxic agents 9. Chronic inflammation, in contrast to acute, is a highly specific response and involves different sets of immune cells, mostly lymphocytes and macrophages. Macrophages, which are derived from monocytes, are multi-functional cells. Their role in the inflammatory response includes elimination of microbes and necrotic tissue; initiation of repair; secretion of many cytokines (including chemokines, interleukins, tumor necrosis factor, and eicosanoids); and interactions with T lymphocytes.
Chronic Inflammation and Cancer
Chronic Inflammation and Cancer
There are a number of cancers which have been associated with chronic inflammatory conditions, as shown in Table 1.

The image above is used with permission. Citation: Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park). 2011;25(5):400-13) http://www.cancernetwork.com/oncology-journal/chronic-inflammation-and-cancer-role-mitochondria
The most notable association is between inflammatory bowel disease (IBD) and the development of colorectal cancer (CRC). Patients with IBD are 5-7 times more likely to develop CRC. Around 4 in 10 patients with ulcerative colitis—a type of IBD—develop CRC after 25-35 years16

Further evidence supporting the link between cancer and inflammation is that drugs that reduce inflammation also reduce the risk of some cancers. Studies investigating the effect of continuous use of non-steroidal anti-inflammatory drugs (NSAIDs) have shown decreased risks of developing certain cancers. NSAIDs are a common class of pain relieving drugs, which include ibuprofen, aspirin, and naproxen. Colon cancer risk is decreased by 50% with continued use (at least 6 months) of non-aspirin NSAIDs and by 40% with continuous long-term use of aspirin.17 NSAIDs work by blocking proteins (called cyclooxygenases or COX) that are known to cause inflammation. 18 Aspirin has also been shown to prevent cancer through a decrease in blood plasma levels of an oncometabolite, 2-Hydroxyglutarate (2HG) (a metabolite is a substance formed during metabolism and the prefix onco means the metabolite is associated with cancer).19 The accumulation of 2HG has been linked with activating an oncogene, MYC.20 This is another mechanism by which aspirin may prevent cancer. Additionally, aspirin lowers risk of death from prostate cancer.21 The United States Preventive Services Task Force recommends "initiating low-dose aspirin use for the primary prevention of cardiovascular disease (CVD) and colorectal cancer (CRC) in adults aged 50 to 59 years who have a 10% or greater 10-year CVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years." 22
It's important to understand that linking things together like this (correlation) does not always mean that one action causes another (causation). The studies mentioned above mostly show a correlation between inflammation and cancer, but do not explicitly show that inflammation causes cancer. Below, you can learn about research being done to identify the mechanisms by which inflammation actually causes cancer.
Does inflammation cause cancer?
The idea of a causal relationship between inflammation and cancer dates back to 1836, when Rudolf Virchow (shown below) hypothesized sites of long-term (chronic) inflammation as the origin of cancer. 18 This relationship is now widely accepted and scientists are bringing to light the cellular and molecular mechanisms behind this connection. Long-term inflammatory conditions increase the risk of cancer by:

1. Causing sustained cell proliferation
2. Increasing the presence of growth factors
3. Causing changes in surrounding cells and proteins creating activated stroma)
4. Leading to the invasion and activation of inflammatory immune cells
5. Increasing the amounts of DNA damaging agents in the area.18
Because cancer is a disease caused by genetic changes, the presence of DNA-damaging chemicals, including reactive oxyen species (ROS) and nitric oxide species (NOS) produced by immune cells are critical in the development of cancer. ROS and NOS are small chemicals (called free radicals) that can directly cause DNA damage. If the damage occurs in oncogenes or tumor suppressors, then the affected cell may begin to divide in an unregulated way, a key to the development of cancer.
Some of the other mechanisms are more elusive. The COX-2 enzyme, produced by immune cells, leads to the production of signaling molecules called prostaglandins, which then cause inflammation. The COX-2 protein has been shown to:
1. lead to genomic instability
2. induce expression of BCL2-mediated resistance to at least one chemotherapy drug (doxorubicin)23 The BCL2 protein prevents apoptosis that should be triggered by abnormal cell activity and chemotherapy drugs.
Another finding supporting this causal relationship is that a deficiency of the anti-inflammatory cytokine, IL-10, results in DNA mutations in a mouse model of IBD.24
Image: "Virchow1" by Hanns Fechner - http://www.kunsttexte.de/download/bwt/werner.pdf Gabriele Werner, Das Bild vom Wissenschaftler - Wissenschaft im Bild, in: kunsttexte.de Seite 2. Licensed under Public Domain via Wikimedia Commons - https://commons.wikimedia.org/wiki/File:Virchow1.JPG#/media/File:Virchow1.JPG
For information about reducing chronic inflammation, check out the section on Inflammation Prevention.
Learn more about oncogenes
Learn more about tumor suppressors
Learn more about BCL2
Learn about NSAIDS, including aspirin, and cancer prevention
Preventing Inflammation
Inflammation is influenced by diet and lifestyle. As far as diet, many foods have been associated with either increased or decreased inflammation. The following list includes some examples, with a brief description of the way that they are thought to work. Note that this list does not mean that we are recommending any specific food or diet.
Foods that have been studied for their anti-inflammatory properties:

- Suppression of NF-kB and STAT3 pathways
- Also exhibits similar activities to tumor necrosis factor blockers, vascular endothelial cell growth factor blockers, human epidermal growth factor receptor blockers, and a HER2 blocker 26
Pomegranate27
- Juice and peel possess antioxidant properties
- Juice, peel and oil can interfere with tumor cell proliferation, cell cycle, invasion and angiogenesis
Extra Virgin Olive Oil (EVOO)28, 29
- Phenolic compound in EVOO called oleocanthal acts as COX inhibitor
- Oleocanthal also can induce apoptosis in cancer cells via lysosomal membrane permeability

Soy
- A compound in soy called genistein can have anti-inflammatory properties by affecting monocytes, granulocytes, and lymphocytes
- In mice, soy protein can inhibit NF-kB and AKT signaling pathways
- Dietary omega-3-fatty acids have anti-inflammatory and immune system modulating factors
- Higher ratios of omega-3-fatty acids to omega-6-fatty acids may decrease oxidative stress
- Garlic consumption leads to inhibition of NF-kB in in vitro studies
- Allicin, a compound in garlic, alleviates inflammation in rats
Ginger35
- Has anti-inflammatory effects in mice
Phytochemicals (these are present in multiple different plants):
Flavenoids36
- Casticin and chrysosplenol D inhibit inflammation in vitro and in vivo in mice
Carotenoids37
- Studies have shown that carotenoids have anti-oxididant and anti-inflammatory properties
Foods that have been linked with INCREASED inflammation include:
Trans fatty acids38
- Possible role in gut inflammation

Red meat 39
- Contains a sugar molecule that may lead to inflammation.
Decreased inflammation has also been associated with mind-body therapies.
Activities linked to decreased inflammation include:
Tai chi40
- Breast cancer survivors* with insomnia who engaged in Tai Chi for three months showed a decrease in inflammatory markers IL-6 and TNF
Yoga41
- Breast cancer survivors* who engaged in yoga showed decreases in IL-6 and TNF
*For more information on life post-cancer, visit our Survivorship page.
Immune System and Inflammation Summary
Immune System Introduction
- The immune system is able to distinguish between 'self' and 'non-self' and between normal and abnormal cells.
- The immune system acts through two broad and somewhat overlapping mechanisms - Specific Immune Responses and Non-Specific (Innate) Immunity.
The Innate Immune System
- The innate immune system carries out the non-specific functions.
- The innate immune system consists of three components:
- Physical and chemical barriers such as skin, mucus, and earwax
- Cells including macrophages and neutrophils
- Proteins that include enzymes found in saliva and tears
- The innate immune system recognizes general features of potential pathogens.
The Acquired Immune Response
- The acquired immune response carries out specific or adaptive immunity.
- The adaptive response develops and changes over the course of our lifetimes.
- The adaptive immune response is highly specific for invading pathogens.
- T cells and B cells are the main cell types of the acquired immune system.
- The specific immune response is characterized by the following: 1) antigen specificity, 2) diversity, 3) memory, and 4) self:non-self discrimination.
- The adaptive immune response can detect cancer cells.
Cells of the Acquired Immune Response
- B cells and T cells are known as lymphocytes and they originate in the bone marrow.
- Lymphocytes reside in lymphatic tissue such as lymph nodes and the spleen.
- A protein or other product that can be recognized by the immune system and lead to the production of an immune response is known as an antigen.
- B cells produce antibodies that bind tightly to a pathogen which is then inactivated or destroyed.
- T cells mature into either helper T cells or cytotoxic T lymphocytes.
The Immune System and Cancer
- The immune system can recognize mutant or otherwise abnormal cells as foreign.
- Cancer cells can mutate enough so that they are able to escape the surveillance mechanisms of the immune system.
- Many cancers produce chemical signals that inhibit the actions of immune cells.
- Some tumors grow in locations such as the eyes or brain, which are not regularly patrolled by immune cells.
- Immunotherapy and cancer vaccines are designed to provide the immune system with the signals that it needs to recognize and destroy cancer cells.
Inflammation Summary
Inflammation is the body’s response to potentially harmful events. It is a protective and necessary process that involves recruiting cells and molecules of the host’s immune system to the site of injury. Inflammation itself can become harmful when the process is prolonged.
Acute (short-term) inflammation is an observable physical response with four key signs:
1. Redness
2. Heat
3. Swelling
4. Pain.
Acute inflammation has not been shown to increase cancer risk.
Chronic (long-term) inflammation does not show the symptoms of acute inflammation. It is a prolonged immune response that often leads to tissue damage. These responses can last for many years. Chronic inflammation is different from acute inflammation, though acute inflammation can develop into chronic inflammation if the injury/infection is long-lasting or if something prevents the normal healing process. Things that can lead to chronic inflammation include persistent infections, hypersensitivity diseases, and long-term exposure to toxic agents. Research also indicates that being overweight/obese can also trigger some aspects of chronic inflammation. 42
A summary of the features of acute and chronic inflammation
Acute
- Fast onset (minutes or hours)
- Recruited immune cells are mostly neutrophils
- Causes mild and self-limiting damage to host tissue
- Noticeable local and systemic physical symptoms (the four cardinal signs listed above. 9
Chronic
- Slower onset (days)
- Involves recruitment of macrophages (derived from monocytes) and lymphocytes
- Causes moderate to severe damage to host tissue
- Chronic inflammatory conditions have been linked to increased risk of cancer. There are several ways that chronic inflammation can cause cancer, including:
1. Causing sustained cell proliferation.
2. Increasing the presence of growth factors.
3. Causing changes in the proteins that surround cells (produces ‘activated’ stroma).
4. Leading to the invasion and activation of inflammatory immune cells.
5. Increasing the amounts of DNA damaging agents in the area.14
There are a number of ways to potentially prevent chronic inflammation and one of the most significant is avoiding being overweight or obese.
If you find the material useful, please consider linking to our website
- 1abcd Charles Janeway, Paul Travers, Mark Walport and Mark Schlomchik. Immunobiology. (2004) 6th Edition. Garland Publishing, NY, NY
- 2 Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., & Reece, J. B. (2017). Campbell Biology (11th ed.). Pearson.
- 3 Gavin P. Dunn, Allen T. Bruce, Hiroaki Ikeda, Lloyd Old and Robert D. Schreiber. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology. (2002) 3 (11): 991-998. [PUBMED]
- 4 Biagi E, Rousseau RF, Yvon E, Vigouroux S, Dotti G and Brenner MK. "Cancer vaccines: dream, reality or nightmare?" Clinical Experimental Medicine. (2002) 2:109-118 [PUBMED]
- 5 Lahmar Q, Keirsse J, Laoui D, Movahedi K, Van Overmeire E, Van Ginderachter JA. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim Biophys Acta. 2015 Jul 2. pii: S0304-419X(15)00052-9. [Epub ahead of print] [PUBMED]
- 6 Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015 Apr;36(4):229-39. Epub 2015 Mar 11. [PUBMED]
- 7 Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009 Mar;9(3):162-74. [PUBMED]
- 8 Katoh H, Watanabe M. Myeloid-Derived Suppressor Cells and Therapeutic Strategies in Cancer. Mediators Inflamm. 2015;2015:159269 Epub 2015 May 19 [PUBMED]
- 9abcdefg Kumar, Vinay, Abul K. Abbas, Jon C. Aster, and Stanley L. Robbins. "Chapter 2 Inflammation and Repair." <i>Robbins Basic Pathology</i>. 9th ed. Philadelphia, PA: Elsevier/Saunders, 2013. 29-74. Print.
- 10 Image: Klaus D. Peter, Gummersbach, Germany (Own work) via Wikimedia Commons (http://creativecommons.org/licenses/by/3.0/de/deed.en)
- 11 Punchard NA, Whelan CJ, Adcock I. The Journal of Inflammation. J Inflamm (Lond) 2004 Sep 27;1(1):1. [PUBMED]
- 12 Ahmad A, Banerjee S, Wang Z, Kong D, Majumdar AP, Sarkar FH. Aging and inflammation: etiological culprits of cancer. Curr Aging Sci. 2009 Dec; 2(3):174-86. [PUBMED]
- 13abcde Emily Shacter and Sigmund A. Weitzman, Chronic Inflammation and Cancer, Colorectal Cancer, Oncology Journal, January 31, 2002
- 14ab Lisa M. Coussens and Zena Werb, Inflammation and Cancer, Nature 420, 860-867, December 19, 2002
- 15 Ryan GB, Majno G., Acute inflammation. A review., Am J Pathol. 1977 Jan;86(1):183-276. [PUBMED]
- 16 Kamp DW, Shacter E, Weitzman SA; Chronic inflammation and cancer: the role of the mitochondria; Oncology (Williston Park). 2011 Apr 30;25(5):400-10, 413. [PUBMED]
- 17 García-Rodríguez LA, Huerta-Alvarez C; Epidemiology. 2001 Jan;12(1):88-93; Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. [PUBMED]
- 18abc Lisa M. Coussens & Zena Werb. Inflammation and cancer. Nature (420). December 2002. [http://osteosarcomasupport.org/immunology/inflammation-cancer-nature-2002.pdf]
- 19 Liesenfeld DB, Botma A, Habermann N, Toth R, Weigel C, Popanda O, Klika KD, Potter JD, Lampe JW, Ulrich CM; Aspirin Reduces Plasma Concentrations of the Oncometabolite 2-Hydroxyglutarate: Results of a Randomized, Double-Blind, Crossover Trial; Cancer Epidemiol Biomarkers Prev. 2016 Jan;25(1):180-7. [PUBMED]
- 20 Terunuma A, Putluri N, Mishra P, Mathé EA, Dorsey TH, Yi M, Wallace TA, Issaq HJ, Zhou M, Killian JK, Stevenson HS, Karoly ED, Chan K, Samanta S, Prieto D, Hsu TY, Kurley SJ, Putluri V, Sonavane R, Edelman DC, Wulff J, Starks AM, Yang Y, Kittles RA, Yfantis HG, Lee DH, Ioffe OB, Schiff R, Stephens RM, Meltzer PS, Veenstra TD, Westbrook TF, Sreekumar A, Ambs S; J Clin Invest. 2014 Jan;124(1):398-412; MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. [PUBMED]
- 21 Assayag J, Pollak MN, Azoulay L.J Urol; 2015 Apr;193(4):1220-5; The use of aspirin and the risk of mortality in patients with prostate cancer. [PUBMED]
- 22 Bibbins-Domingo K, on behalf of the U.S. Preventive Services Task Force. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 12 April 2016
- 23 Singh B, Cook KR, Vincent L, Hall CS, Berry JA, Multani AS, Lucci A; J Surg Res. 2008 Jun 15;147(2):240-6; Cyclooxygenase-2 induces genomic instability, BCL2 expression, doxorubicin resistance, and altered cancer-initiating cell phenotype in MCF7 breast cancer cells. [PUBMED]
- 24 T SCHEININ, D M BUTLER, F SALWAY, B SCALLON, and M FELDMANN; Clin Exp Immunol. 2003 Jul; 133(1): 38¿43; Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis [PUBMED]
- 25 Kim JH, Gupta SC, Park B, Yadav VR, Aggarwal BB, Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-¿B and NF-¿B-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis, Mol Nutr Food Res. 2012 Mar;56(3):454-65 [PUBMED]
- 26ab Aggarwal BB, Sundaram C, Malani N, Ichikawa H.; Curcumin: the Indian solid gold.; Adv Exp Med Biol. 2007;595:1-75. [PUBMED]
- 27 Lansky EP, Newman RA; Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer; J Ethnopharmacol. 2007 Jan 19;109(2):177-206. [PUBMED]
- 28 Onica LeGendreab, Paul AS Breslincd & David A Fosterae; (-)-Oleocanthal rapidly and selectively induces cancer cell death via lysosomal membrane permeabilization; Molecular & Cellular Oncology, Volume 2, Issue 4, 2015
- 29 Lucas L, Russell A, Keast R.; Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal.; Curr Pharm Des. 2011;17(8):754-68. [PUBMED]
- 30 Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Malarkey WB, Hwang BS, Blackburn E.Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav Immun. 2013 Feb;28:16-24 [PUBMED]
- 31 Mori TA, Beilin LJ. Curr Atheroscler Rep. 2004 Nov;6(6):461-7. Omega-3 fatty acids and inflammation. [PUBMED]
- 32 Georgia Schäfer and Catherine H. Kaschula; The Immunomodulation and Anti-Inflammatory Effects of Garlic Organosulfur Compounds in Cancer Chemoprevention; Anticancer Agents Med Chem. 2014 Feb; 14(2): 233¿240. [PUBMED]
- 33 Li C, Lun W, Zhao X, Lei S, Guo Y, Ma J, Zhi F. Allicin alleviates inflammation of trinitrobenzenesulfonic acid-induced rats and suppresses P38 and JNK pathways in Caco-2 cells. Mediators Inflamm. 2015;2015:434692. [PUBMED]
- 34 Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R; Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial; Brain Behav Immun. 2012 Aug;26(6):988-95. [PUBMED]
- 35 Hsiang CY, Cheng HM, Lo HY, Li CC, Chou PC, Lee YC, Ho TY; J Agric Food Chem. 2015 Jul 8;63(26):6051-8; Ginger and Zingerone Ameliorate Lipopolysaccharide-Induced Acute Systemic Inflammation in Mice, Assessed by Nuclear Factor-¿B Bioluminescent Imaging. [PUBMED]
- 36 Li YJ, Guo Y, Yang Q, Weng XG, Yang Y, Wang YJ, Chen Y, Zhang D, Li Q, Liu XC, Kan XX, Chen X, Zhu XX, Kmoníèková E, Zídek Z; Toxicol Appl Pharmacol. 2015 Aug 1;286(3):151-8; Flavonoids casticin and chrysosplenol D from Artemisia annua L. inhibit inflammation in vitro and in vivo. [PUBMED]
- 37 Kaulmann A, Bohn T; Nutr Res. 2014 Nov;34(11):907-29; Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. [PUBMED]
- 38 Ann M. Bode and Zigang Dong; Chapter 7 The Amazing and Mighty Ginger Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Benzie IFF, Wachtel-Galor S, editors. Boca Raton (FL): CRC Press/Taylor & Francis; 2011.
- 39 Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda K1 Gregg CJ, Bingman AE, Secrest P, Diaz SL, Varki NM, Varki A. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):542-7. Epub 2014 Dec 29. [PUBMED]
- 40 Michael R. Irwin,corresponding author Richard Olmstead, Elizabeth C. Breen, Tuff Witarama, Carmen Carrillo, Nina Sadeghi, Jesusa M. G. Arevalo, Jeffrey Ma, Perry Nicassio, Patricia A. Ganz, Julienne E. Bower, and Steve Cole. Tai Chi, Cellular Inflammation, and Transcriptome Dynamics in Breast Cancer Survivors With Insomnia: A Randomized Controlled Trial. J Natl Cancer Inst Monogr. 2014 Nov; 2014(50): 295-301.
- 41 Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, Emery CF, Layman R, Mrozek EE, Glaser R. Yoga's impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014 Apr 1;32(10):1040-9. [PUBMED]
- 42 Calle EE, Kaaks R., Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms, Nat Rev Cancer. 2004 Aug;4(8):579-91 [PUBMED]

