Diagnostic techniques, like MRI, Ultrasound, CT, or PET, are very useful but in some cases when suspicious tissue (a lesion) is discovered, a doctor may want to get a sample of the suspected cancer. Removal of a sample is called a biopsy.
Some other 'biopsies' only require giving a sample of blood or other bodily product (urine, saliva, etc.).
This page contains information on:
- Tissue Biopsies
- Liquid Biopsies
This video explains both fine needle aspiration (FNA) and core needle biopsy (CNB), two types of biopsies that are explained in the following paragraphs.
Types of Tissue Biopsies
A tissue biopsy is the removal of either a portion of a lesion (incisional) or the entire lesion (excisional). The tissues are then sent to a lab where a pathologist will diagnose the sample.
There are several different types of biopsy. The type used depends on the goal of the biopsy (i.e. remove the entire lesion or obtain a small sample), the cancer type and the location of the cancer.
1. Incisional Biopsy
An incisional biopsy removes only a portion of a suspected tumor.1 This technique is used when a lesion is too large to remove entirely or when the location of the tumor would result in unacceptable amounts of scarring. Incisional biopsies may require local anesthesia and may or may not require stitches.
2. Excisional Biopsy
An excisional biopsy removes the entire tumor and some surrounding tissue.1 If a diagnosis of cancer results, the biopsy will have removed the entire tumor. An excisional biopsy is done using local anesthesia and is the most invasive of all the biopsy techniques. The wound may need to be stitched closed or a skin graft may be needed. These biopsies usually produce a scar.
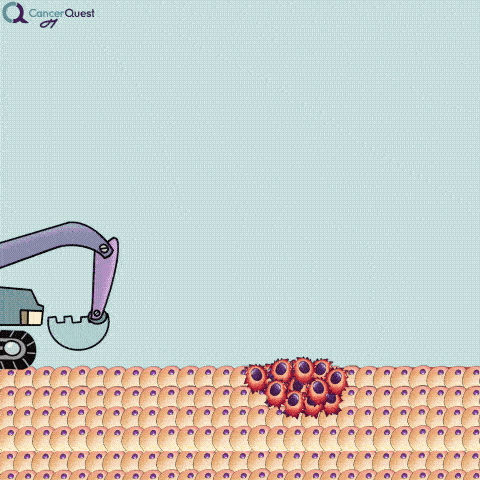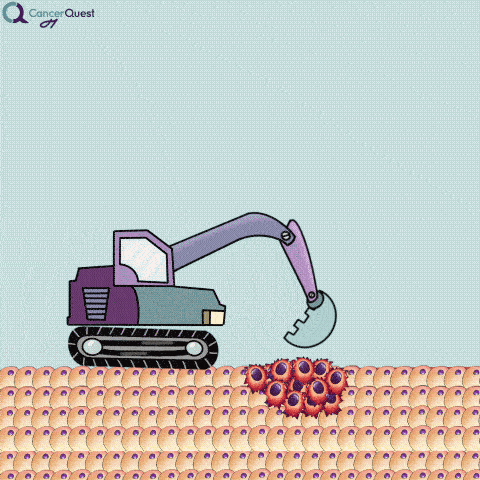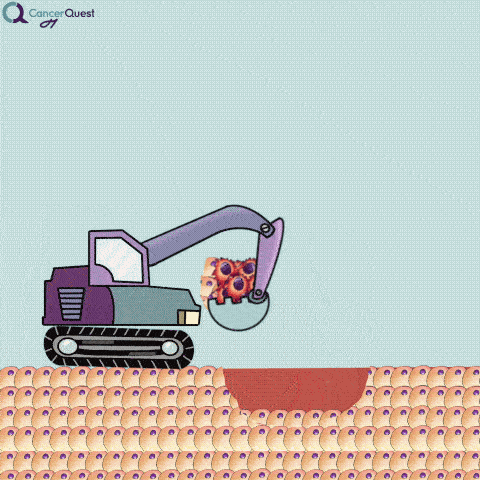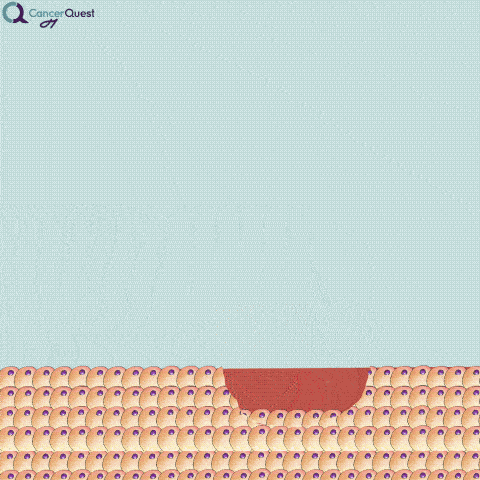
An excisional biopsy removes the entire tumor (red cells) and nearby healthy tissue (tan cells).
3. Types of excisional and incisional biopsies.
-
Punch
A punch biopsy is used to collect a deep sample of skin and is usually used for large lesions or lesions on the palm, sole, finger/toe, face, and ear. This technique removes a portion of skin approximately ¼ inch deep and may result in a scar.
-
Shave
A shave biopsy removes the epidermis and a small portion of the dermis, the top two layers of the skin. This technique uses a surgical blade or razor to shave off a portion of the skin. This procedure is easy on a patient because it is relatively pain free (done under local anesthesia) and requires no stitches. Shave biopsies are not normally used for suspected melanoma because the cut is not deep enough to allow measurement of the depth to which the lesion has spread.
-
Needle
A needle biopsy is rarely used to obtain skin tissue; it is usually used to remove a sample from internal organs, lymph nodes, or deep skin areas. These techniques involve the use of a small, hollow needle and is sometimes aided by an imaging technique such as x-ray. There are two types of needle biopsy, fine needle aspiration (FNA) and core needle biopsy. They differ in the amount of tissue removed. Core needle biopsies remove a larger tissue sample than FNA.1 More about these below.
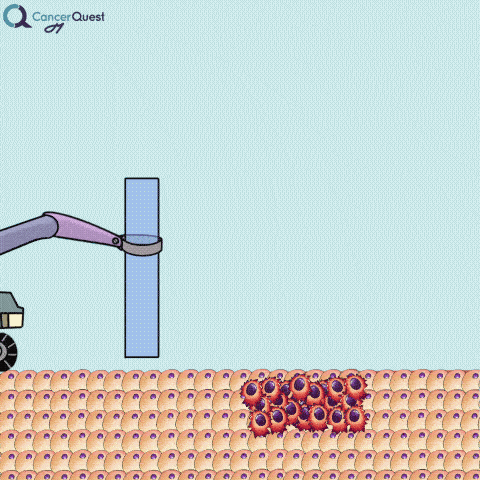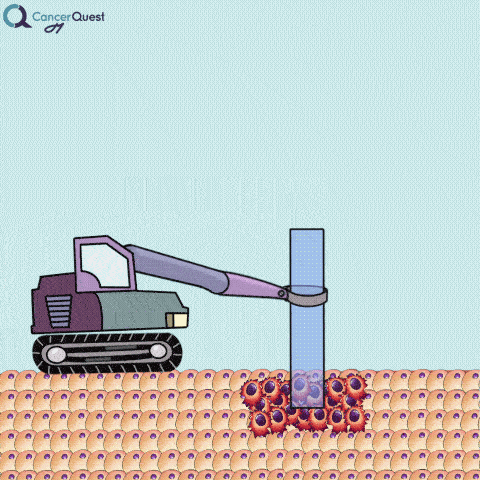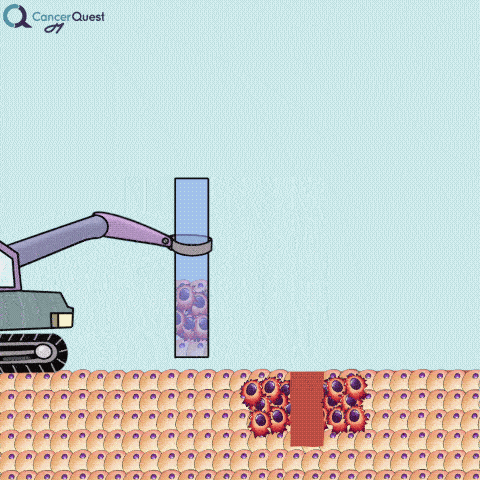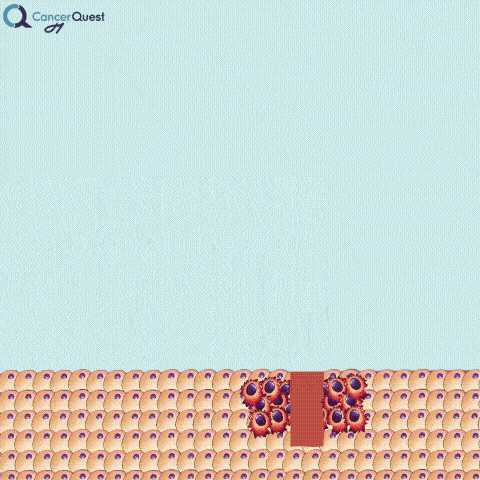
Needle biopsies are used to take samples of a suspected tumor (red cells). Normal cells (tan) may also be collected. They can differ in the type of needle used and the number of samples taken.
Fine Needle Aspiration (FNA)
Procedure
FNA is done with a small, 20-27 gauge needle (same size or smaller than most needles used in ordinary blood test, the larger the gauge the smaller the needle). The area is sterilized with alcohol to prevent infection. The needle is then inserted and aimed at the center of the lesion. When the needle reaches the lesion a very small piece is removed by suction. This is repeated to ensure that a proper amount of usable sample is obtained. Local anesthesia is not used in most cases because the sting from a local anesthesia needle is more painful than the FNA procedure itself. If the lesion is non-palpable the doctor may use ultrasound or other imaging techniques to help guide the needle precisely into the suspicious lesion.2
Analysis
Samples are sent to a pathologist specially trained in cytology (cellular abnormalities) to be processed and interpreted. The samples are placed on glass slides and stains are used to reveal the details of the cells. The diagnosis will generally come back as one of five options:2, 3
- Benign - the mass is not of much concern and will not cause any significant problems as long as it remains unchanged.
- Atypically indeterminate - a diagnosis cannot be obtained from the sample. Other tests are needed to determine the nature of the lesion.
- Suspicious/probably malignant - not a diagnosis of cancer. This type of diagnosis requires additional investigation because the sample has abnormal characteristics. This lesion should be biopsied with a more complete method to determine whether a malignancy (cancer) is present.
- Malignant - a diagnosis of cancer; should be biopsied and tested for exact tumor makeup to prepare for treatment.
- Unsatisfactory - a diagnosis cannot be determined from the sample because of insufficient sample size, processing or other machine or human errors.
The Triple Test (TT) Method in Breast Cancer
After the biopsy is diagnosed by a pathologist all aspects of the case should be considered by a clinician, this is called the triple test method or TT. The triple test method considers the results of the physical examination, imaging (mammography, MRI, etc.) results and the cellular (cytological) findings of the pathologies (based on the biopsy samples). When all of these aspects are considered, a FNA is very accurate. The false positive and false negative rates are similar to biopsies obtained by more invasive surgeries. The TT method should always be used to diagnosis a breast mass using FNA.2
Preparation and Side Effects
There is no special preparation for a FNA, no fasting, special diet, etc. In almost all cases FNA will be done in the doctor's office and not in a hospital. There is very little pain associated with a FNA and the procedure is very safe, resulting in only a little bruising and tenderness around the biopsy area.
Core Needle Biopsy
Biopsies are samples of tissue that are removed for closer examination.
Procedure
Core needle biopsy (CNB) is similar to a fine needle aspiration (FNA), except that a larger (11-18 gauge) needle is used and the pathology report is different. Because the needle is larger than in a FNA, local anesthesia is used to numb the area before insertion. A small nick in the skin is made and the doctor inserts the needle through this nick. At least three, usually more, samples are taken from each breast mass to ensure an adequate sample. In most cases the doctor will use an imaging technique, such as ultrasound, to help guide the needle into the desired tissue. Steri-Strips™ are used to close the small cut and a larger bandage is placed on top to protect the wound.4
Analysis
A core biopsy sample is studied differently than a FNA sample. The larger size of the sample allows the pathologist to look at the way groups of cells are organized instead of looking at individual cells. In CNB a trained pathologist looks for changes associated with a variety of diseases.4 Because cancer cells are dividing in an abnormal fashion, they make the tissue around them appear disorganized. By examining collections of cells (tissue) instead of individual cells, pathologists get a good sense for the health of the organ from which the sample was removed. The study of tissues is called histology and the study of abnormal tissues is called histopathology.
Preparation and Side Effects
You should limit alcohol consumption the day before and do not take any products containing aspirin 5 days before the procedure because alcohol and aspirin can thin the blood and result in excessive bleeding. After the procedure there may be discomfort, bruising, and bleeding. These will be short term and pain can be managed with pain medications (except aspirin). The bandages covering the wound will need to be changed and the wound cleaned.
FNA vs Core Biopsy
Below is a table comparing Fine Needle Aspiration and Core Needle Biopsy.2, 5, 6
|
|
Core Needle Biopsy (CNB) |
|
| Sample Removed | Removes only a very small portion of the lesion | Removes a small portion in most cases, occasionally removes the entire lesion |
| Needle Size | 22-27 gauge | 11-18 gauge |
| Pathology Type | Cytopathology | Histopathology |
| Interpretation Time | Immediately | Delayed |
| Diagnostic Abilities | Limited ability to specifically diagnose benign lesions No ability to differentiate between in situ and invasive breast cancer |
Strong ability to specifically diagnose benign lesions. Some ability to differentiate between in situ and invasive breast cancer. |
| Disadvantages | Cannot be used for additional study | More invasive, time consuming, expensive |
| Advantages | Inexpensive, quick, readily available, and very safe | Can be used for additional study and has more specific diagnostic abilities than FNA |
| Effectiveness | Sensitivity: 75.8-98.7% Specificity: 60-100% Positive Predictive Value: 93.5-100% |
Sensitivity: 91-99.6%% Specificity: 98-100% Positive Predictive Value: 100% |
Watch a video about Sensitivity and Specificity of Medical Tests
Frequently Asked Questions about Biopies
General Biopsy Questions
Questions About Fine Needle Aspirations
Questions About Core Needle Biopsy
Liquid Biopsies
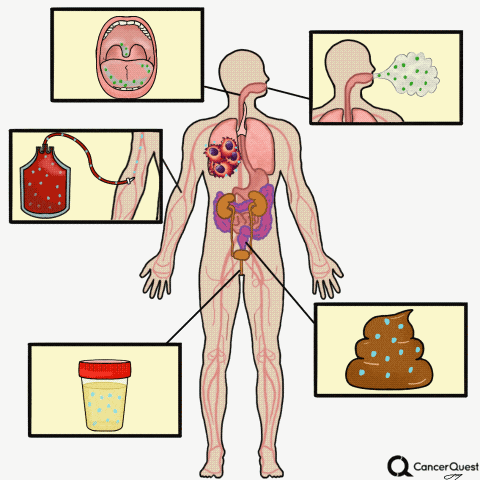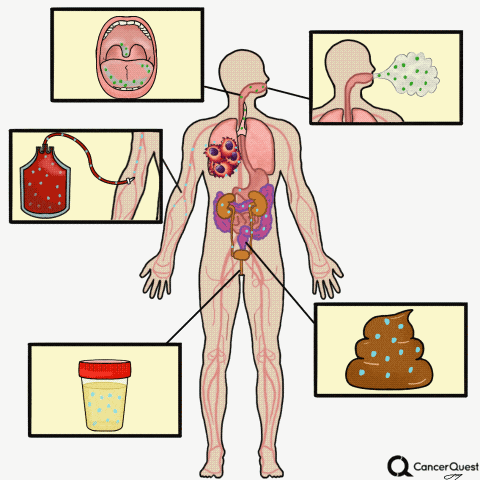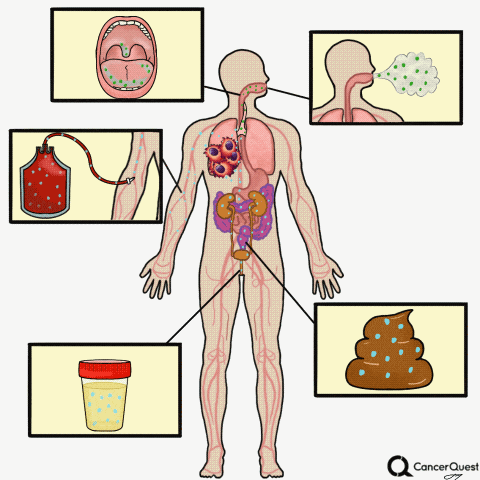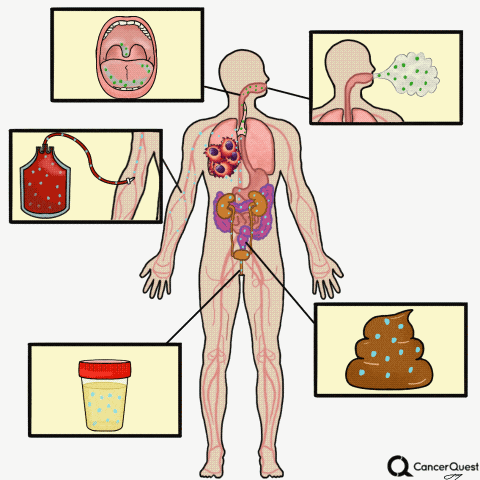
Type the caption here.
Currently in the works is a method to accurately screen for (and track) cancer safely, quickly, cheaply, and non-invasively: liquid biopsies. Although liquid biopsies are not yet routine in the clinic (right now, physicians rely on tissue samples) 7, there are many companies (including Illumina’s GRAIL, Johnson & Johnson, Pathway Genomics, Epic Sciences, Guardant Health, and many others) developing blood-based cancer tests.
Liquid biopsies require only a small sample of blood. Traditional biopsies can be painful, costly, time-consuming, and potentially risky for the patient.8 The biomarkers that can found in a liquid biopsy include circulating tumor cells (CTCs), tumor DNA (specifically referred to as “cell-free circulating tumor DNA” or ctDNA) and RNA fragments that have been shed from tumor cells. There’s also exosomes, tiny bubble-like structures (vesicles) released from cells. Exosomes can carry a variety of proteins, DNA, and RNA throughout the body.9
Liquid biopsies can be used to detect and stage cancer. They are also likely to be used to track the response of patients to cancer treatments. A large amount of information can be gained by looking at the different disease-specific items found in blood. Liquid biopsies provide scientists and clinicians a way to learn about the genetic defects in a tumor. They can see what tumor suppressor genes are broken, what oncogenes are activated, changes in the number of copies of genes, or abnormal epigenetic alterations (i.e. methylation) of cancer-associated genes. 10.
| Biomarkers found in liquid biopsies | Description |
| Circulating Tumor Cells (CTCs) | Cells can break off from tumors and enter the bloodstream, where they are referred to as CTCs. If they stop and grow at a site distant from the primary tumor, they will have metastasized 9. CTCs are rare and difficult to isolate 11. CTC detection has been associated with worse outcomes for patients with early breast cancer 12. |
| Cell-free circulating tumor DNA (ctDNA) | DNA can be released into the bloodstream from tumor cells dying from apoptosis or necrosis 13. It may also be released by living tumor cells and CTCs 14. ctDNA can be single-stranded or double-stranded, and the pieces are different lengths. Importantly, ctDNA carries the mutations found in the tumor 14 and can be used to create mutation profiles of the cancer. ctDNA analysis has been shown to be more sensitive at detecting mutations than CTCs 10. |
| Cell-free microRNA (miRNA) | miRNAs are tiny, non-protein-coding nucleic acid sequences (about 19-22 nucleotides long). They regulate the transcription of genes 15. miRNA can be released by tumor cells into the bloodstream, and research shows that they can alter gene expression in cells far from where they originated 16. Studies suggest that the types of miRNAs released is tissue and cancer specific. This makes miRNA profiling from liquid biopsy samples a promising method for detecting cancer and tracking treatment responses 15. |
| Exosomes | Exosomes are tiny vesicles (~30-100 nm in diameter) that move through the bloodstream and carry proteins, RNA, DNA. They function in cell-to-cell communication 17. Exosomes can be isolated from blood samples and analyzed for biomarkers specific to cancer 18. |
| DNA Methylation | Looking at epigenetic changes, specifically at methylation patterns, on circulating DNA is another way of detecting cancer 19. Tumor suppressor genes may be silenced by the addition of methyl groups (-CH3) to specific parts of their DNA sequence. |
Currently, liquid biopsies can’t conclusively tell whether or not a patient has a tumor somewhere (a traditional tissue biopsy would be the only way to be completely sure). They are likely to be very useful in helping physicians decide whether or not a more invasive tissue biopsy would be wise. This is especially important when testing sites like the brain and lungs, where traditional biopsies can be dangerous/harmful. Also, by including liquid biopsies in routine blood screens, doctors may be able to detect evidence of cancer in asymptomatic individuals early in cancer progression 13, and they can take the necessary steps to treat it before it becomes advanced/widespread.
Another benefit of a liquid biopsy deals with the issue of tumor heterogeneity.20 Almost all cancers start out as one defective cell. Over time, and many cell divisions, the subpopulations of daughter cells gain mutations and become different from one another; the cancer cells in a single tumor are similar to each other, but not identical. Cancer evolves. During a traditional biopsy, a physician cuts out a few tiny sections of the (suspected) tumor. Cells in another, (un-biopsied) part of that same tumor may be slightly different, and may even respond to treatment differently. Liquid biopsies have the potential to reveal details about cells from all parts of the tumor, and a provide a more complete picture of what genes that particular tumor relies on.
Conducting liquid biopsies for tumor-specific mutations should be useful post-treatment as a way to gauge how successful a treatment is working/worked.13 Detection of ctDNA in the blood after surgery could identify patients who will need additional treatment.20 Also, one reason relapse occurs is because a tumor develops resistance to the therapies being used; a treatment works for a while, but the few cancer cells that aren’t affected by the drug will proliferate and take over. Physicians could detect this resistance earlier by screening for common resistance mutations in the ctDNA floating around in patients’ blood.20 This would help guide drug selection for additional treatments.
Liquid biopsies for cancer detection/tracking aren’t yet being used regularly. The tests have to be standardized and validated with large numbers of samples; there are multiple methods of extracting and analyzing tumor cells, cell-free nucleic acids, and exosomes from a blood sample, and different testing methods may give different results. Plus, biomarkers in liquid samples will also need to be validated as reliable and cancer-specific in large-scale clinical trials, and these need to be compared to traditional tissue biopsies before using them in the clinic.15 Because ctDNA is found at very low levels in blood, the approved tests will have to be very sensitive.10
Liquid Biopsy (Blood test) Research Results:
- A 2018 study demonstrated that that liquid collected by Pap tests could also be used to detect endometrial and ovarian cancers. Dubbed PapSEEK by the researchers, this test was shown to detect the majority of endometrial cancers and many ovarian cancers from existing patients. The detection was improved by taking samples closer to the source of the endometrial/ovarian cells. Work is ongoing to improve the sensitivity of the test. The test dis not show ANY false positives in this group of women.21
- In a 2016 study, researchers from the Dana-Farber Cancer Institute took 180 patients with non-small cell lung carcinoma (NSCLC), 120 of whom had been recently diagnosed, and 60 of whom had relapsed after their initial treatments. The cell-free DNA in the blood samples of the patients were tested for mutations in two genes that are commonly mutated in NSCLC (the EGFR and KRAS genes). Each patient also had a standard tissue biopsy, and the results of the two tests were compared. Liquid biopsies were faster; the turnaround time for results was about 3 days for the liquid biopsy versus 12 days for the new patients and 27 days for the patients whose tumors were drug-resistant. Analyzing the cell-free DNA in the blood was also just as accurate as the conventional biopsy, and the liquid test was even able to catch some EGFR resistance mutations that the conventional test missed.22
- In 2015, researchers from the Memorial Sloan Kettering Cancer Center discovered that they could analyze the free-floating tumor DNA in liquid biopsies to predict how breast cancer patients would respond to certain treatments. They looked at blood samples from 587 patients, some of whom were receiving the hormone drug fulvestrant plus a placebo, and the rest who were receiving fulvestrant plus buparlisib, a drug that blocks a pathway that can promote resistance to hormone therapies (the PI3K pathway). Patients who had a mutation in the PIK3CA gene (a mutation which activates that PI3K pathway) dramatically benefitted from the combination treatment compared to those who received a placebo (7 months of progression-free survival versus 3.2 months).23 With the information obtained from liquid biopsies, doctors could identify patients who wouldn’t benefit from certain drugs and can help them avoid any unnecessary side effects.
- Researchers at the University of Texas M. D. Anderson Cancer Center completed a study in 2015 in which they extracted exosomes from the blood of three patients with pancreaticobiliary cancers (two pancreatic, one ampullary) and analyzed the DNA and RNA gene sequences inside the exosomes. They were able to obtain the same information they would have from a tissue biopsy (an invasive procedure for a visceral organ like the pancreas). They were able to characterize the tumor mutations, gene copy-number changes, possible treatment responses, and targets for immunotherapies.24
- A paper published in The Lancet Oncology in 2015 compared data from liquid and tissue biopsies in metastatic colorectal cancer patients. The isolated DNA from the blood of patients to look for KRAS, PIK3CA, and BRAF mutations. They also quantified how much DNA there was, and examined the levels of 15 different proteins. The researchers saw that the anti-angiogenesis drug regorafenib benefited certain groups of patients based on what mutations they had and the concentrations of certain proteins—data that could be collected from blood samples. The blood test could also predict clinical outcomes and detect new mutations that the tumors had acquired since the initial tissue biopsies.25
Other Types of ‘Liquid’ Biopsies: Saliva, Sputum, Breath, Urine, Stool
Sputum is a thick mixture of saliva, mucus and other material that can be brought up from the throat and air passages leading to the lungs. Sputum contains cells and DNA and can be examined for abnormalities, including some that indicate the presence of lung cancer.28, 29 Although it is not a very sensitive test, examination of cells in sputum is still used to detect lung cancer. In part because of the poor sensitivity of the test, routine screening for lung cancer via sputum samples is not recommended.28
Recent advances have improved the test considerably. The ThinPrep® preparation technique has been shown to provide additional sensitivity.30 Research is now being performed to assess the value of looking at fluorescence in body fluids to detect cancer31 and to look for the presence of genetic defects and specific genes that could indicate the presence of cancer.32, 33
- Bladder cancer: This is already being used in the clinic. Physicians can check for cancer or pre-cancerous cells in the urine, see if there is any blood in the urine (a possible first sign of bladder cancer), or check for the levels of proteins linked to cancer such as NMP22, carcinoembryonic antigen (CEA), and bladder tumor-associated antigen (BTA).37
- Prostate cancer: A 2016 study identified four different messenger RNAs (mRNAs) that may serve as biomarkers for prostate cancer by comparing mRNA levels in urine samples of patients with and without prostate cancer.38
- Kidney cancer: A study from 2015 showed that physicians could look at the levels of two proteins, aquaporin-1 and perlipin-2, which are elevated in kidney cancer.39
- Pancreatic cancer: The possibility of a urine test for pancreatic cancer, which is often too late in tumor progression to be effectively treated, is also being studied. A 2015 study published in Clinical Cancer Research reported on a three-protein biomarker panel that could be used to detect patients who had early-stage pancreatic cancer.40 The researchers looked at the levels of different proteins in 18 patients: six controls, six with chronic pancreatitis, and six with pancreatic ductal adenocarcinoma. Three of these proteins (LYVE1, REG1A, and TFF1) were elevated in cancer patients compared to the healthy patients and those with pancreatitis. Using urine samples, the researchers were able to predict stage 1 and 2 pancreatic cancer with over 90% accuracy.
Stool tests:
A stool (fecal) DNA test is considered a type of liquid biopsy.
- Colorectal cancer: A stool test to detect colorectal cancer was approved by the FDA in 2014 and covered by Medicare.41 This test, called Cologuard® detects elevated levels of mutant DNA from cancer cells, and also detects hemoglobin (a chemical found in blood). This fecal DNA test is an improvement upon the previous fecal immunochemical test (FIT), which only checks for blood in the stool. The Cologuard® sample can also be easily collected at home and sent off for analysis in a prepaid box. Though false-positives limit the test’s accuracy, it is much less invasive than a colonoscopy. Learn more about false-positive and negative medical tests.
- 1abc Declan, Walsh et al. Palliative Medicine. 1st ed. Philadelphia, PA: Saunders/Elsevier, 2009.
- 2abcd A Abati and A Simisir. Breast fine needle aspiration biopsy: Prevailing Recommendations and contemporary practices. Clinics in Laboratory Medicine. 2005; 25: 631-654. [PUBMED]
- 3 EM Tani, L Skoog, T Lowhagen. Clinical Utility of Fine-Needle Aspiration Cytology of the Thyroid. Annual Review of Medicine. 1988; 39:255-260.
- 4ab BD Florentine, CJ Cobb, K Rankle, T Greaves, SE Martin. Core needle biopsy. A useful adjunct to fine-needle aspiration in select patients with palpable breast lesions. Cancer Cytopathology. 1997; 81:33-39. [PUBMED]
- 5 ES de Paredes, TG Langer, J Cousins. Interventional Breast Procedures. Current Problems in Diagnostic Radiology. 1998; September/October: 138-184. [PUBMED]
- 6 B Chaiwun and P Thorner. Fine needle aspiration for evaluation of breast masses. Current Opinion in Obstetrics and Gynecology. 2007; 19: 48-55. [PUBMED]
- 7 Larrea, Erika, Carla Sole, Lorea Manterola, Ibai Goicoechea, María Armesto, María Arestin, María Caffarel, Angela Araujo, María Araiz, Marta Fernandez-Mercado, and Charles Lawrie. "New Concepts in Cancer Biomarkers: Circulating MiRNAs in Liquid Biopsies." IJMS International Journal of Molecular Sciences 17.5 (2016): 627. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4881453/]
- 8 Karachaliou N, Mayo-de-las-Casas C, Molina-Vila MA, Rosell R. "Real-time liquid biopsies become a reality in cancer treatment". Ann Transl Med 2015;3(3):36. doi: 10.3978/j.issn.2305-5839.2015.01.16 [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4356857/]
- 9ab Brock, Graham, Elena Castellanos-Rizaldos, Lan Hu, Christine Coticchia, and Johan Skog. "Liquid Biopsy for Cancer Screening, Patient Stratification and Monitoring." Translational Cancer Research 4.3 (2015). [http://tcr.amegroups.com/article/view/4546/4921]
- 10abc Heitzer, E., P. Ulz, and J. B. Geigl. "Circulating Tumor DNA as a Liquid Biopsy for Cancer." Clinical Chemistry 61.1 (2014): 112-23. [http://www.ncbi.nlm.nih.gov/pubmed/25388429] [PUBMED]
- 11 Denis, Jérôme Alexandre, Alexia Patroni, Erell Guillerm, Dominique Pépin, Naoual Benali-Furet, Janine Wechsler, Gilles Manceau, Maguy Bernard, Florence Coulet, Annette K. Larsen, Mehdi Karoui, and Jean-Marc Lacorte. "Droplet Digital PCR of Circulating Tumor Cells from Colorectal Cancer Patients Can Predict KRAS Mutations before Surgery." Molecular Oncology (2016). [http://www.ncbi.nlm.nih.gov/pubmed/27311775] [PUBMED]
- 12 Ignatiadis, Michail, Brigitte Rack, Francoise Rothé, Sabine Riethdorf, Charles Decraene, Hervé Bonnefoi, Christian Dittrich, Carlo Messina, Melanie Beauvois, Elisabeth Trapp, Theodora Goulioti, Konstantinos Tryfonidis, Klaus Pantel, Madeline Repollet, Wolfgang Janni, Martine Piccart, Christos Sotiriou, Saskia Litiere, and Jean-Yves Pierga. "Liquid Biopsy-based Clinical Research in Early Breast Cancer: The EORTC 90091-10093 Treat CTC Trial." European Journal of Cancer 63 (2016): 97-104. [http://www.ncbi.nlm.nih.gov/pubmed/27289552] [PUBMED]
- 13abc Crowley, Emily, Federica Di Nicolantonio, Fotios Loupakis, and Alberto Bardelli. "Liquid Biopsy: Monitoring Cancer-genetics in the Blood." Nature Reviews Clinical Oncology Nat Rev Clin Oncol 10.8 (2013): 472-84. [http://www.ncbi.nlm.nih.gov/pubmed/23836314] [PUBMED]
- 15abc Endzeli¿š, Edgars, Vita Melne, Zane Kalni¿a, Vilnis Lietuvietis, Una Rieksti¿a, Alicia Llorente, and Aija Lin¿. "Diagnostic, Prognostic and Predictive Value of Cell-free MiRNAs in Prostate Cancer: A Systematic Review." Molecular Cancer Mol Cancer 15.1 (2016). [http://www.ncbi.nlm.nih.gov/pubmed/27189160] [PUBMED]
- 16 Larrea, Erika et al. New Concepts in Cancer Biomarkers: Circulating miRNAs in Liquid Biopsies. Ed. William Chi-shing Cho. International Journal of Molecular Sciences 17.5 (2016): 627. PMC. Web. 20 June 2016. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4881453/#!po=24.0741]
- 17 Zhang, Xuan, Zenglin Pei, Jinyun Chen, Chunxia Ji, Jianqing Xu, Xiaoyan Zhang, and Jin Wang. "Exosomes for Immunoregulation and Therapeutic Intervention in Cancer." J. Cancer Journal of Cancer 7.9 (2016): 1081-087. [http://www.ncbi.nlm.nih.gov/pubmed/27326251] [PUBMED]
- 18 Isola, Allison L., and Suzie Chen. ¿Exosomes: The Link between GPCR Activation and Metastatic Potential?¿ Frontiers in Genetics 7 (2016): 56. PMC. [http://www.ncbi.nlm.nih.gov/pubmed/27092178] [PUBMED]
- 19 Tomasetti, Marco, Monica Amati, Jiri Neuzil, and Lory Santarelli. "Circulating Epigenetic Biomarkers in Lung Malignancies: From Early Diagnosis to Therapy." Lung Cancer (2016). [http://www.sciencedirect.com/science/article/pii/S0169500216303506]
- 20abc Esposito, Angela, Carmen Criscitiello, Marzia Locatelli, Monica Milano, and Giuseppe Curigliano. "Liquid Biopsies for Solid Tumors: Understanding Tumor Heterogeneity and Real Time Monitoring of Early Resistance to Targeted Therapies." Pharmacology & Therapeutics 157 (2016): 120-24. [http://www.ncbi.nlm.nih.gov/pubmed/26615782] [PUBMED]
- 21 Wang Y, Li L, Douville C, Cohen JD, Yen TT, Kinde I, Sundfelt K, Kjær SK, Hruban RH, Shih IM, Wang TL, Kurman RJ, Springer S, Ptak J, Popoli M, Schaefer J, Silliman N, Dobbyn L, Tanner EJ, Angarita A, Lycke M, Jochumsen K, Afsari B, Danilova L, Levine DA, Jardon K, Zeng X, Arseneau J, Fu L, Diaz LA Jr, Karchin R13, Tomasetti C, Kinzler KW, Vogelstein B, Fader AN, Gilbert L, Papadopoulos N. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med. 2018 Mar 21;10(433). pii: eaap8793. doi: 10.1126/scitranslmed.aap8793. [PUBMED]
- 22 Dana-Farber Cancer Institute. "'Liquid biopsy' blood test detects genetic mutations in common form of lung cancer." ScienceDaily. ScienceDaily, 7 April 2016. <www.sciencedaily.com/releases/2016/04/160407115907.htm>. [https://www.sciencedaily.com/releases/2016/04/160407115907.htm]
- 23 Memorial Sloan Kettering Cancer Center. "Potential of liquid biopsy for breast cancer patients: Simple blood test could help tailor treatments for advanced breast cancer patients." ScienceDaily. ScienceDaily, 11 December 2015. <www.sciencedaily.com/releases/2015/12/151211145041.htm>. [https://www.sciencedaily.com/releases/2015/12/151211145041.htm]
- 24 University of Texas M. D. Anderson Cancer Center. "Pancreas cancer liquid biopsy flows from blood-borne packets of tumor genes." ScienceDaily. ScienceDaily, 17 December 2015. [https://www.sciencedaily.com/releases/2015/12/151217115230.htm]
- 25 Vall d'Hebron Institute of Oncology. "Liquid biopsy identifies mutations in colorectal cancer undetected in tissue biopsy." ScienceDaily. ScienceDaily, 13 July 2015. [https://www.sciencedaily.com/releases/2015/07/150713205420.htm]
- 26 Wong, David. "2016 AAAS Annual Meeting (February 11-15, 2016) February 10 - 15, 2016." Abstract: Saliva Liquid Biopsy for Cancer Detection (2016 AAAS Annual Meeting (February 11-15, 2016)). [https://aaas.confex.com/aaas/2016/webprogram/Paper16195.html]
- 27 Nagler, R. "Concomitant Analysis of Salivary Tumor Markers--A New Diagnostic Tool for Oral Cancer." Clinical Cancer Research 12.13 (2006): 3979-984. [http://www.ncbi.nlm.nih.gov/pubmed/16818695] [PUBMED]
- 28ab Mascaux C, Peled N, Garg K, Kato Y, Wynes MW, Hirsch FR. Early detection and screening of lung cancer. Expert Rev Mol Diagn. 2010 Sep;10(6):799-815. [PUBMED]
- 29 Thunnissen FB. Sputum examination for early detection of lung cancer. J Clin Pathol. 2003 Nov;56(11):805-10. [PUBMED]
- 30 Choi YD, Han CW, Kim JH, Oh IJ, Lee JS, Nam JH, Juhng SW, Park CS. Effectiveness of sputum cytology using ThinPrep method for evaluation of lung cancer. Diagn Cytopathol. 2008 Mar;36(3):167-71. [PUBMED]
- 31 Al-Salhi M, Masilamani V, Vijmasi T, Al-Nachawati H, Vijayaraghavan AP. Lung Cancer Detection by Native Fluorescence Spectra of Body Fluids-A Preliminary Study. J Fluoresc. 2010 Oct 19. [Epub ahead of print] [PUBMED]
- 32 Varella-Garcia M, Schulte AP, Wolf HJ, Feser WJ, Zeng C, Braudrick S, Yin X, Hirsch FR, Kennedy TC, Keith RL, Barón AE, Belinsky SA, Miller YE, Byers T, Franklin WA. The detection of chromosomal aneusomy by fluorescence in situ hybridization in sputum predicts lung cancer incidence. Cancer Prev Res (Phila). 2010 Apr;3(4):447-53. Epub 2010 Mar 23. [PUBMED]
- 33 Jiang FF, Todd N, Li R, Zhang H, Fang HB, Stass SA. A panel of sputum-based genomic marker for early detection of lung cancer. Cancer Prev Res (Phila). 2010 Sep 23. [Epub ahead of print] [PUBMED]
- 34 Amal, Haitham, Marcis Leja, Konrads Funka, Roberts Skapars, Armands Sivins, Guntis Ancans, Inta Liepniece-Karele, Ilze Kikuste, Ieva Lasina, and Hossam Haick. "Detection of Precancerous Gastric Lesions and Gastric Cancer through Exhaled Breath." Gut 65.3 (2015): 400-07. [http://gut.bmj.com/content/early/2015/03/09/gutjnl-2014-308536.abstract]
- 35 Geetha D. Vallabhaneni, Sheryl G. A. Gabram, Kichun Sky Lee, Taofeek Kunle Owonikoko, Johann Christoph Brandes, Scott Arthur Kono, Nabil F. Saba, Fadlo Raja Khuri, Suresh S. Ramalingam, Charlene W. Bayer. "Breath analysis for early detection of lung cancer." J Clin Oncol 30 June 2012 [http://meetinglibrary.asco.org/content/98210-114]
- 36 Krilaviciute, Agne et al. ¿Detection of Cancer through Exhaled Breath: A Systematic Review.¿ Oncotarget 6.36 (2015): 38643¿38657. [http://www.ncbi.nlm.nih.gov/pubmed/26440312] [PUBMED]
- 37 "Can bladder cancer be found early?" American Cancer Society. 2016. [http://www.cancer.org/cancer/bladdercancer/detailedguide/bladder-cancer-detection]
- 38 Mengual, Lourdes et al. ¿Using Gene Expression from Urine Sediment to Diagnose Prostate Cancer: Development of a New Multiplex mRNA Urine Test and Validation of Current Biomarkers.¿ BMC Cancer 16 (2016): 76. PMC. Web. 11 July 2016. [http://www.ncbi.nlm.nih.gov/pubmed/26856686] [PUBMED]
- 39 Morrissey, Jeremiah J. et al. ¿Evaluation of Urine Aquaporin 1 and Perilipin 2 Concentrations as Biomarkers to Screen for Renal Cell Carcinoma.¿ JAMA oncology 1.2 (2015): 204¿212. PMC. [http://www.ncbi.nlm.nih.gov/pubmed/26181025] [PUBMED]
- 40 Radon, Tomasz P et al. ¿Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma.¿ Clinical cancer research¿: an official journal of the American Association for Cancer Research 21.15 (2015): 3512¿3521. [http://www.ncbi.nlm.nih.gov/pubmed/26240291] [PUBMED]
- 41 Issa, I., & Noureddine, M. (2017). Colorectal cancer screening: An updated review of the available options. World journal of gastroenterology, 23(28), 5086–5096. http://doi.org/10.3748/wjg.v23.i28.5086 (Original work published 2017年7月) [PUBMED]
