Also called the Papanicolaou test, it was first developed by G. N. Papanicolaou and his colleagues in the 1940s. A pap test can detect pre-cancerous and cancerous cells in the vagina and cervix. The cervix is the lower part of the uterus that connects the vagina with the main part of the uterus.
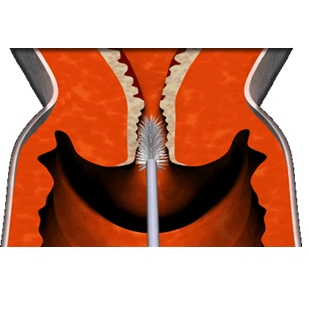
In a Pap test a small sample of cells is removed from the cervix with a brush or spatula. The image below shows the process. The procedure is not painful as only the surface of the cervix is touched. The cells are then smeared onto a slide and examined under a microscope. A Pap test can identify abnormal cells and detect changes before cancer has fully developed.1.2
The direct smear method has been used for a long time, and is relatively inexpensive. Cell samples are placed directly on a glass slide and sent to the lab. New developments have made the test more efficient, but they are significantly more expensive. It has not been determined yet if the new methods increase detection sensitivity enough to warrant the increased cost. The two new methods are liquid-based cytology, in which the cell sample is fixed with a special liquid before being placed on the slide to make the cervical cells more visible and automated detection of abnormal cells utilizing a machine.1
In 2018 researchers showed that liquid collected by Pap tests could also be used to detect endometrial and ovarian cancers. The test was used to identify cancer in existing patients. Next they will try to detect cancers in women who have not yet been diagnosed with cancer. Although the test does not detect every case, it is an exciting start because there are no other detection tests for early ovarian and endometrial cancers. It is also non-invasive - a type test called liquid biopsy.3
Learn more about liquid biopsies and cancer.
Cervical cancer usually develops slowly from pre-cancerous cells. By detecting changes early, treatment can be used to prevent cervical cancer from developing. From 1955 to 1992, death from cervical cancer decreased 74%, largely due to the pap smear.4
There are certain risk factors that place a woman at higher risk for cervical cancer. Not all women with these risk factors develop cervical cancer, but there is very little chance of developing the disease if they are avoided. They include:
- Human Papillomavirus (HPV) infection: There are many different forms of this virus, and some are associated with the development of genital warts and other forms are associated with the development of cancer. HPV may be passed from one person to another through sexual contact, and condoms are not completely effective in preventing infection.
- Smoking: Cancer-causing substances from cigarettes have been found in the cervical mucus of women who smoke, placing these women at a much higher risk of cervical cancer development.
- Human Immunodeficiency Virus (HIV) infection: Because this virus lowers the immune system of those infected, they may develop cancer more quickly than normal.
Other factors such as age and family history can not be avoided, but should be taken into account when considering testing.
For more details, please view the section on cervical cancer under Cancer by Type.
- 1ab Vince A, Lepej SZ. Diagnostic methods and techniques in cervical cancer prevention Part II: Molecular diagnostics of HPV infection. Med Glas Ljek komore Zenicko-doboj kantona. 2010 Feb;7(1):18-25. [PUBMED]
- 2 Behtash N, Mehrdad N. Cervical cancer: screening and prevention. Asian Pac J Cancer Prev. 2006 Oct-Dec;7(4):683-6. [PUBMED]
- 3 Wang Y, Li L, Douville C, Cohen JD, Yen TT, Kinde I, Sundfelt K, Kjær SK, Hruban RH, Shih IM, Wang TL, Kurman RJ, Springer S, Ptak J, Popoli M, Schaefer J, Silliman N, Dobbyn L, Tanner EJ, Angarita A, Lycke M, Jochumsen K, Afsari B, Danilova L, Levine DA, Jardon K, Zeng X, Arseneau J, Fu L, Diaz LA Jr, Karchin R13, Tomasetti C, Kinzler KW, Vogelstein B, Fader AN, Gilbert L, Papadopoulos N. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med. 2018 Mar 21;10(433). pii: eaap8793. doi: 10.1126/scitranslmed.aap8793. [PUBMED]
- 4 "Pap Test." American Cancer Society October 2010. [http://www.cancer.org/Cancer/CervicalCancer/MoreInformation/CervicalCancerPreventionandEarlyDetection/cervical-cancer-prevention-and-early-detection-find-pre-cancer-changes]
