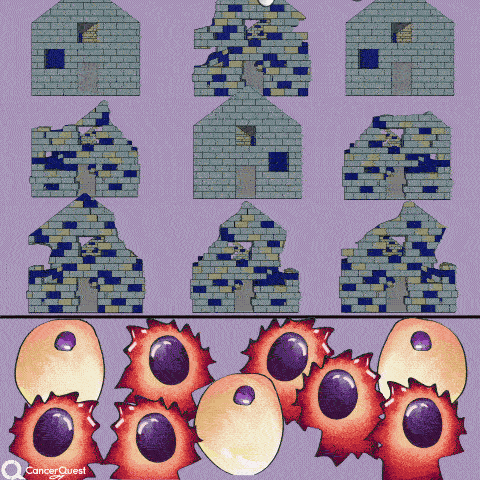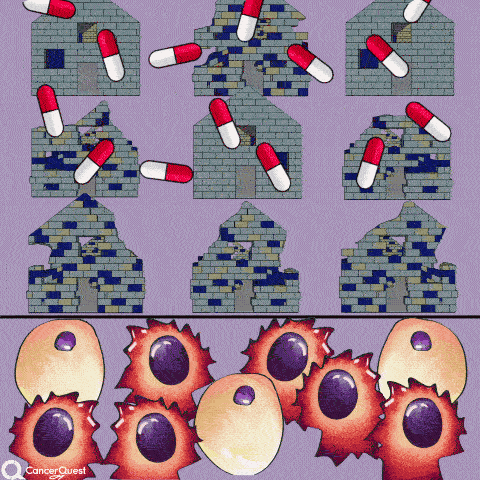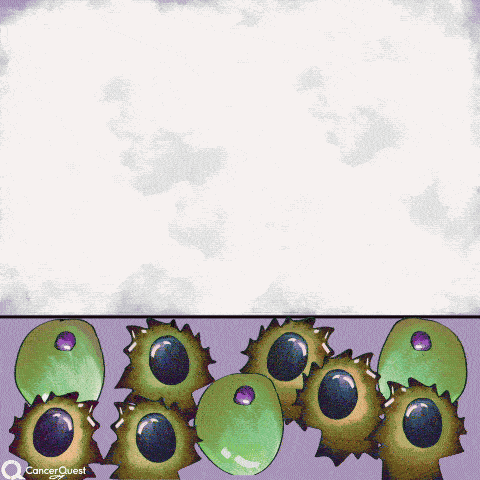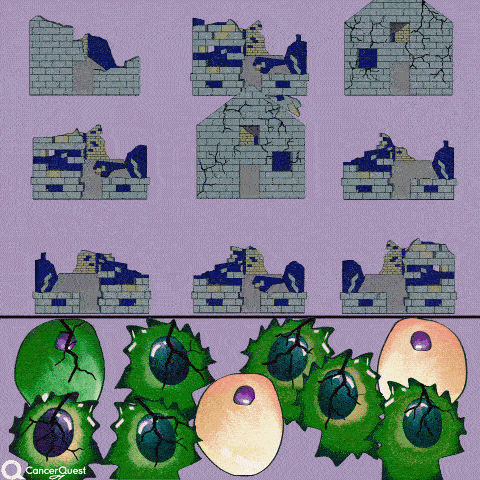
The term chemotherapy, or chemo., refers to a wide range of drugs used to treat cancer. These drugs usually work by killing dividing cells. Since cancer cells have lost many of the regulatory functions present in normal cells, they will continue to attempt to divide when other cells do not. This trait makes cancer cells susceptible to a wide range of cellular poisons.
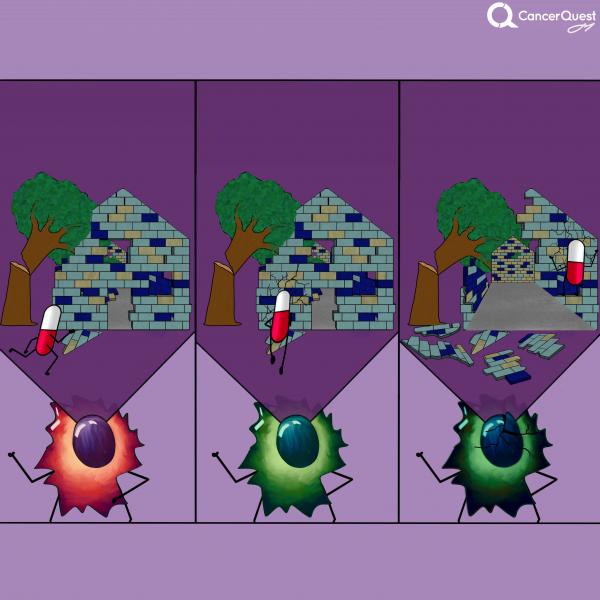
Many chemotherapy drugs kill cancer cells by causing damage to DNA (their genes). Since cancer cells are already damaged, this extra push can kill them.
The chemotherapy agents work to cause cell death in a variety of ways. Some of the drugs are naturally occurring compounds that have been identified in various plants and some are man-made chemicals. A few different types of chemotherapy drugs are briefly described below. For more information on a particular type of drug, choose from the list below.
- Antimetabolites: Drugs that interfere with the formation of key biomolecules within the cell. These drugs ultimately interfere with cell division.
- Folate Antagonists: These are also known as antifolates, inhibit dihydrofolate reductase (DHFR), an enzyme involved in the formation of nucleotides. When this enzyme is blocked, nucleotides are not formed, which disrupts DNA replication and cell division
- Purine Antagonists: Chemicals used to build the nucleotides of DNA and RNA
- Pyrimidine Antagonists: Drugs that act as decoys that block the synthesis of pyrimidine containing nucleotides
- Genotoxic Drugs: Drugs that damage DNA. By causing DNA damage, these agents interfere with DNA replication, and cell division.1
- Spindle Inhibitors: These agents prevent proper cell division by interfering with the cytoskeletal components that enable one cell to divide into two.1
- Additional Chemotherapy Agents: These agents inhibit cell division by mechanisms that are not covered in the three categories listed above.
- Glossary of Chemotherapy Agents: An easy to use table of chemotherapy drugs including trade name, generic name, and type. With links to more information.
Normal cells are more resistant to the drugs because they often stop dividing when conditions are not favorable. Normal cells are also better at repairing their DNA than cancer cells. Not all normal dividing cells escape however, a fact that contributes to the toxicity of these drugs. Cell types that are normally rapidly dividing, such as those in the bone marrow (making blood cells) and in the lining of the intestine, tend to be hardest hit. Death of the normal cells produces some of the common side-effects of chemotherapy, including hair loss, anemia, immune suppression and stomach/digestive problems.
Watch more patient interviews.
Antimetabolites
In order to understand antimetabolites and how they work, it is necessary to briefly discuss the processes that are being targeted by these agents. The term metabolism refers to the many chemical reactions that take place in our bodies. We are constantly breaking down food into usable components and using those components to build our proteins, DNA and other cellular structures. Metabolite is a general term for the organic compounds that are synthesized, recycled, or broken down in cells. Materials that provide us with key metabolites enter our body as food. These compounds can be broken down into simpler structures that can be re-used in our cells. Examples include vitamins and amino acids. Metabolites that are the end products of a process or pathway may be excreted by the body. An example is urea, the end product of protein metabolism, excreted by the body as a component of urine.
Antimetabolites are structurally similar to metabolites, but they cannot be used by the body in a productive manner. In the cell, antimetabolites are mistaken for the metabolites they resemble, and are processed in the cell in a manner analogous to the normal compounds. The presence of the 'decoy' antimetabolites prevents the cells from carrying out vital functions and the cells are unable to grow and survive. Many of the antimetabolites used in the treatment of cancer interfere with the production of the nucleic acids, RNA and DNA.2 If new DNA cannot be made, cells are unable to divide.
There are several different cellular targets for antimetabolites. Some common classes of antimetabolites are:
How Antimetabolites Work: 5-FU
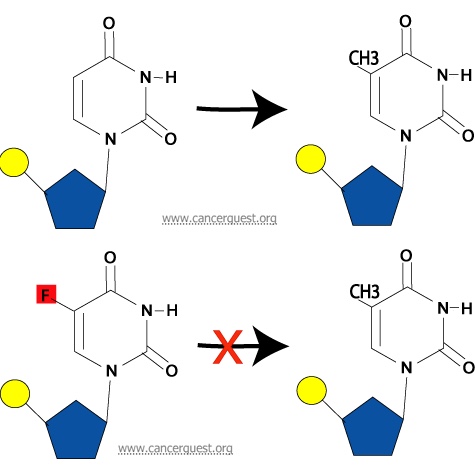
Interaction with Thymidylate Synthase: A final way in which 5-FU may inhibit normal DNA synthesis is its ability to prevent the synthesis of thymine nucleotides from uracil nucleotides. Thymine differs from uracil by the presence of a methyl group (a one-carbon unit) on the 5th carbon in the pyrimidine ring. This methyl group is added on by an enzyme called thymidylate synthase. If a 5-FU molecule is in the nucleotide instead of a uracil, the enzyme cannot add a methyl group to the 5th carbon due to the fluoride atom at that location in 5-FU. This alteration from the normal pyrimidine gives the drug its name. The addition of methyl is required for the conversion of the uracil nucleotide into the thymine nucleotide, and without this step, the thymine nucleotides can not be made and are not available for DNA synthesis.
Shown below is the normal process (top) and the inhibtion of the process by 5-FU (bottom).
Folate Antagonists
Folate antagonists, also known as antifolates, inhibit dihydrofolate reductase (DHFR), an enzyme involved in the formation of nucleotides. When this enzyme is blocked, nucleotides are not formed, which disrupts DNA replication and cell division. Methotrexate is the primary folate antagonist used as a chemotherapeutic agent. It may be used alone or in combination with other anticancer drugs.
Drug Discovery
In 1948, it was found that a diet with reduced levels of folic acid led to a decrease in leukemia cell counts. That discovery started the search for folate antagonists. The same year, a folate antagonist, aminopterin, was found to produce remissions in childhood leukemias. Methotrexate was discovered soon after, and it proved to be a more effective, less toxic folate analogue. Since then, and despite the isolation of multiple other folate antagonists, methotrexate maintains its significant role as a treatment for breast cancer, osteogenic sarcoma, and leukemias. 3
How Folate Antagonists Work: Methotrexate
Folic acid is a growth( )factor that provides single carbons to the precursors used to form the nucleotides used in the synthesis of DNA and RNA. Folate antagonists, also known as antifolates, act by blocking the active site of dihydrofolate reductase (DHFR), an enzyme that reduces folic acid to its active form. Active folates are co-enzymes necessary for methylation in various metabolic processes, in which they deliver methyl groups (one-carbon units) to specific target molecules. The inhibition of the dihydrofolate reductase keeps the folic acid in an inactive state. A decrease in the amount of activated folates is thought to cause a decrease in methylation, inhibiting a necessary step in purine and thymidylate formation. When nucleic( )acid formation is compromised because of a lack of nucleotides, cell growth is disrupted. 4 Methotrexate is the most commonly used folate antagonist.
More Details:
Folic acid, the core structure of all folates, is not useful until it is chemically reduced. The enzyme that reduces folic acid is dihydrofolate reductase (DHFR). First, DHFR reduces the folic acid into dihydrofolate. Then, DHFR reduces dihydrofolate into tetrahydrofolate (active folate). It is this compound that is used as a donor of methyl groups. The methyl groups are attached to N-5 and/or N-10 of the tetrahydrofolate which carries the methyl groups to other compounds. The enzyme thymidylate synthase (TS) catalyzes transfer of the carbon from the tetrahydrofolate to the target molecules. In order to do so, TS must oxidize the folate ring of the tetrahydrofolate, which reverts it back into a dihydrofolate. For this process to repeat, cells must repeatedly use DHFR to reduce the dihydrofolate into the active tetrahydrofolate form. This requires continual DHFR activity.
Methotrexate inhibits the activity of DHFR by tightly, though reversibly, binding to it rendering it inactive. It enters the cell via specific folate receptors, the low pH folate transporter, or by reduced folate carriers. Once in the cell, methotrexate binds to DHFR. This binding reduces the amount of DHFR available to the cell, and stops the reduction of the tetrahydrofolate precursors, ie. folic acid and dihydrofolic acid. Without tetrahdryofolate, the active folate, the cell cannot create new purine and thymidine nucleotides for DNA synthesis. Without replication, cell growth is blocked.4
Examples of folate antagonists:
Methotrexate
Pemetrexed (Alimta®)
Purine Antagonists
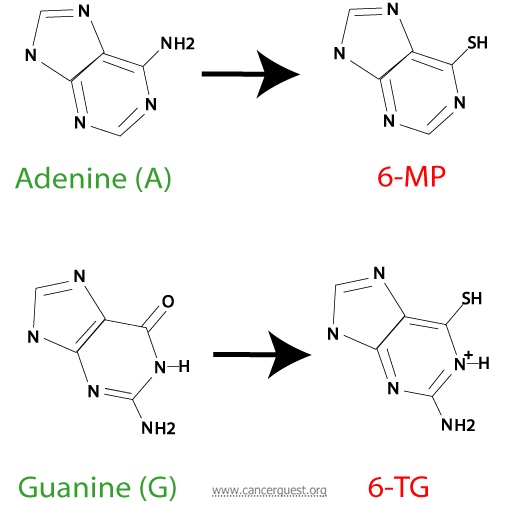
The purines (adenine and guanine) are chemicals used to build the nucleotides of DNA and RNA. The other class of base, the pyrimidines, are represented in DNA by thymine and cytosine and in RNA by cytosine and uracil.
Before a cell can divide it must duplicate its DNA content, so that each daughter cell has a complete and identical set of genetic information. The duplication process (replication) is like an assembly line, during which nucleotides are joined to each other to form the new DNA molecule. Phosphate groups and sugar molecules are joined together to create the long strands of DNA found in our chromosomes. The incorporation of a purine antagonist prevents the continued growth of the DNA and prevents cell division.5
The purine antagonists function by inhibiting DNA synthesis in two different ways:
-
They can inhibit the production of the purine containing nucleotides, adenine and guanine. If a cell doesn't have sufficient amounts of purines, DNA synthesis is halted and the cell cannot divide.5
-
They may be incorporated into the DNA molecule during DNA synthesis. The presence of the inhibitor is thought to interfere with further cell division.5
One of the best known purine antimetabolite is acyclovir, an antiviral agent used to treat herpesvirus infections. Purine antagonists currently used to treat cancer patients include 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG). These drugs are similar to each other, and work in the same way. The structures the normal purines (adenine and guanine) with their antagonists (6-MP and 6-TG) are shown below.
Many times cancers become less sensitive, or resistant, to the drugs that are used to treat them.
Learn about resistance to cancer drugs.
Some purine antagonists used to treat cancer:
Pyrimidine Antagonists
The pyrimidine antagonists act to block the synthesis of pyrimidine containing nucleotides (C and T in DNA; C and U in RNA). The drugs used to block the construction of these nucleotides have structures that are similar to the natural compound. By acting as 'decoys', these drugs can prevent the production of the finished nucleotides. They may exert their effects at different steps in that pathway and may directly inhibit crucial enzymes. The pyrimidine antagonist may also be incorporated into a growing DNA chain and lead to termination of the process.
For a cell to reproduce, it must first faithfully replicate all of the DNA in its genome. During DNA synthesis, pyrimidine and purine molecules must be made available to allow for the synthesis of the nucleotide building blocks and ultimately the new DNA molecules. A reduction in the availability of the raw materials needed to build DNA, which can be caused by pyrimidine antagonists, leads to stoppage of DNA synthesis and inhibition of cell division.
Cancer cells are often quite rapidly dividing and therefore engaged in DNA synthesis. RNA synthesis is necessary for protein production. The pyrimidine antagonists inhibit the normal processes of DNA and/or RNA synthesis.
Some pyrimidine antagonists used in cancer therapy are:
Genotoxic Drugs
Genotoxic drugs are chemotherapy agents that affect nucleic acids and alter their function. These drugs may directly bind to DNA or they may indirectly lead to DNA damage by affecting enzymes involved in DNA replication.2Rapidly dividing cells are particularly sensitive to genotoxic agents because they are actively synthesizing new DNA. If enough damage is done to the DNA of a cell it will often undergo apoptosis, the equivalent of cellular suicide.
The genotoxic chemotherapy treatments include:
- Alkylating agents: The first class of chemotherapy agents used. These drugs modify the bases of DNA, interfering with DNA replication and transcription and leading to mutations.2
- Intercalating agents: These drugs wedge themselves into the spaces between the nucleotides in the DNA double helix. They interfere with transcription, replication and induce mutations.
- Enzyme inhibitors: These drugs inhibit key enzymes, such as topoisomerases, involved in DNA replication inducing DNA damage.2
The goal of treatment with any of these agents is the induction of DNA damage in the cancer cells. DNA damage, if severe enough, will induce cells to undergo apoptosis, the equivalent of cellular suicide. The genotoxic chemotherapy drugs affect both normal and cancerous cells. The selectivity of the drug action is based on the sensitivity of rapidly dividing cells, such as cancer cells, to treatments that damage DNA.2The mode of action also explains many of the side effects of treatment with these drugs. Rapidly dividing cells, such as those that line the intestine or the stem cells in bone marrow, are often killed along with the cancer cells.2In addition to being cytotoxic (cell poisons), these drugs are also mutagenic (cause mutations) and carcinogenic (cause cancer). Treatment with these drugs carries with it the risk of secondary cancers, such as leukemia. These drugs are used to treat a variety of solid cancers and cancers of blood cells, often in combination with other drugs.2
How do Alkylating Agents Work?
Alkylating agents work in three different ways. Aall of them achieve the same end result - disruption of DNA function and cell death.
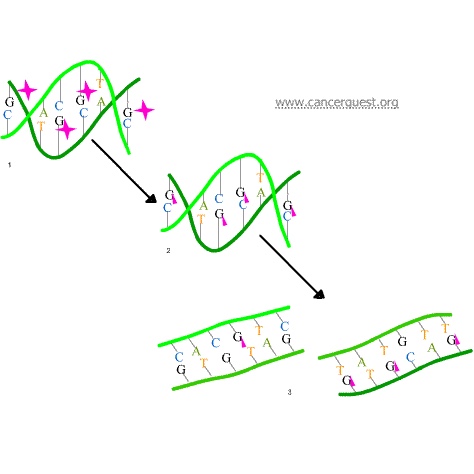
In the first mechanism an alkylating agent (represented in the figure below as a pink star) attaches alkyl groups (small carbon compounds-depicted as pink triangles) to DNA bases. This alteration results in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases (frame 3 of the diagram below). Alkylated bases prevent DNA synthesis and RNA transcription from the affected DNA.
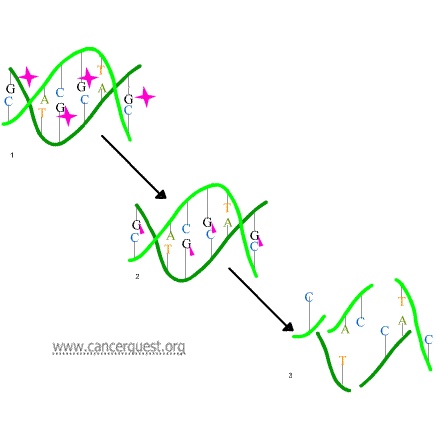
A second mechanism by which alkylating agents cause DNA damage is the formation of cross-bridges, bonds between atoms in the DNA (pink linkages below). In this process, two bases are linked together by an alkylating agent that has two DNA binding sites. Bridges can be formed within a single molecule of DNA (as shown below) or a cross-bridge may connect two different DNA molecules. Cross-linking prevents DNA from being separated for synthesis or transcription.
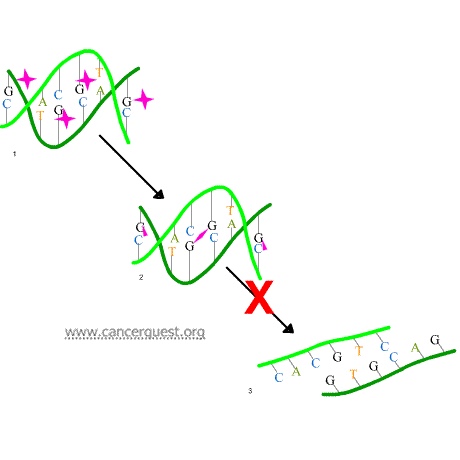
The third mechanism of action of alkylating agents is the induction of mispairing of the nucleotides leading to mutations. In a normal DNA double helix, A always pairs with (is across from) T and G always pairs with C. As the figure below shows, alkylated G bases may erroneously pair with Ts. If this altered pairing is not corrected it may lead to a permanent mutation.
Get more information about some genotoxic chemotherapy drugs:
Busulfan
Bendamustine
Carboplatin
Chlorambucil
Cisplatin
Cyclophosphamide
Daunorubicin
Decitabine
Doxorubicin
Epirubicin
Etoposide
Idarubicin
Ifosfamide
Irinotecan
Lomustine
Mechlorethamine
Melphalan
Mitomycin C
Mitoxantrone
Oxaliplatin
Temozolomide
Topotecan
Spindle Inhibitors
The spindle inhibitors include several different chemotherapy drugs. Unlike the previous drugs discussed, these agents do not work to alter DNA structure or function. These drugs interfere with the mechanics of cell division.
During mitosis, the DNA of a cell is replicated and then divided into two new cells. The process of separating the newly replicated chromosomes into the two forming cells involves spindle fibers. These fibers are constructed with microtubules. These spindle microtubules attach to the replicated chromosomes and pull one copy to each side of the dividing cell, as shown in the animation below. Without functional spindle fibers or spindles, the cell cannot divide and will eventually die. The spindle inhibitor drugs function in a cell-cycle dependent manner, halting division during early mitosis.6
The microtubules that makeup spindle fibers are formed by long chains of smaller subunits of proteins called tubulins. The process of microtubule growth (polymerization) is shown below. As seen in the animation, the process is dynamic and the tubulin subunits can add to or fall off of the microtubule.
Certain types of spindle inhibitors bind to the tubulin monomers and stop the microtubules from being made. By forming a complex with the tubulin monomers, they halt the proper formation of spindle microtubules and disable the movement of chromosomes during mitosis.
Most spindle inhibitors affect both cancerous cells and normal cells and can cause unwanted side effects.
The Vinca alkaloids are a subset of drugs that are derived from the periwinkle plant, Catharanthus roseus (also Vinca rosea, Lochnera rosea, and Ammocallis rosea). It is also commonly called the Madagascar periwinkle or the rose periwinkle. While it has been historically used to treat numerous diseases, it has most recently been employed for its anti-cancer properties. The plant grows in warm regions of the world and especially in the Southern United States. The "flower" is usually pale pink with a dark violet dot in the center.7, 8
All vinca alkaloids are administered intravenously (IV). After injection, they are eventually metabolized by the liver and excreted. They work in a cell-cycle specific manner, halting mitosis of affected cells and causing cell death. As stated in the introduction, the mechanism involves binding to the tubulin monomers and keeping the microtubules (spindle fibers) from forming. Although the plant has medical uses, it can produce many serious side effects if smoked or ingested.
Examples of drugs that interfere with microtubule acitivity.
- Taxanes
- Epothilones
- Vinca Alkaloids
The Discovery of Paclitaxel (Taxol®)
Dr. Monroe E. Wall and Dr. Mansukh C. Wani discovered paclitaxel (Taxol®) at the Research Triangle Institute in 1967. They isolated the compound from the Pacific Yew tree (Taxus brevifolia) and tested it as an anti-tumor drug in rodents. The mechanism of action for paclitaxel was reported by scientists at the Albert Einstein Medical College in 1980. In December of 1992, the FDA approved paclitaxel for the treatment of ovarian cancer. Today the drug is used for a variety of cancers, including ovarian, breast, small-cell and large-cell lung cancers, and Kaposi's sarcoma.9 Paclitaxel is a plant alkaloid that is derived from the bark of the Pacific Yew Tree (see photo). The Pacific Yew grows in moist soils and can be found in British Columbia, Alaska, California, Idaho, Montana, Oregon, and Washington. It takes about 2g of paclitaxel (about 3-10 trees) to treat one patient. Paclitaxel is administered as a series of intravenous injections.10

Image source: Wikimedia Commons
Additional Chemotherapy Agents
While many of the commonly used chemotherapy agents fit into one of the three previously described groupings (genotoxic, spindle inhibitors, and anti-metabolite), some of them work through mechanisms that do not neatly fit into one of these categories. Additionally, some drugs may not have a clearly defined mechanism of action. Some drugs that fall into these categories:
Arsenic trioxide (Trisenox®)
Hydroxyurea
Streptozocin
- 1ab Hurley LH. "DNA and its associated processes as targets for cancer therapy." Nat Rev Cancer (2002). 2(3): 188-200. [PUBMED]
- 2abcdefg Walsh, Declan. Palliative Medicine. 1st ed. Philadelphia: Saunders, 2009.
- 3 Kaye SB. "New antimetabolites in cancer chemotherapy and their clinical impact." Br J Cancer. 1998;78 Suppl 3:1-7. Review. [PUBMED]
- 4ab Matherly LH. "Molecular and cellular biology of the human reduced folate carrier." Prog Nucleic Acid Res Mol Biol. 2001;67:131-62. Review. [PUBMED]
- 5abc Physician's Desk Reference, 56th ed., 2016. Medcial Economics: Thomson Healthcare.
- 6 Schwartz AL, Ciechanover A. "The ubiquitin-proteasome pathway and pathogenesis of human diseases." Annu Rev Med (1999). 50:57-74. [PUBMED]
- 7 Martino E, Casamassima G, Castiglione S, et al. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg Med Chem Lett. 2018;28(17):2816–2826. doi:10.1016/j.bmcl.2018.06.044 [PUBMED]
- 8 Nejat N, Valdiani A, Cahill D, Tan YH, Maziah M, Abiri R. Ornamental exterior versus therapeutic interior of Madagascar periwinkle (Catharanthus roseus): the two faces of a versatile herb. ScientificWorldJournal. 2015;2015:982412. doi:10.1155/2015/982412 [PUBMED]
- 9 Zhang, D., Yang, R., Wang, S., & Dong, Z. (2014). Paclitaxel: new uses for an old drug. Drug Design, Development and Therapy, 8, 279–84. http://doi.org/10.2147/DDDT.S56801 (Original work published December 2014) [PUBMED]
- 10 Sally J. DeNardo, Gerald L. DeNardo, Arutselvan Natarajan, Laird A. Miers, Allan R. Foreman, Cordula Gruettner, Grete N. Adamson, and Robert Ivkov. "Thermal Dosimetry Predictive of Efficacy of 111In-ChL6 Nanoparticle AMF Induced Thermoablative Therapy for Human Breast Cancer in Mice." Journal Nuclear Medicine 2007 48: 437-444. [PUBMED]