Blood consists of three types of cells and cell fragments floating in a liquid called plasma. These cellular components are:
- Red Blood Cells ("erythrocytes," "RBCs") - oxygen-carrying cells
- White Blood Cells ("leukocytes," "WBCs") - cells that help make up the body's immune system
- Platelets ("thrombocytes") - fragments of cells that play an important role in formation of blood clots
The total number of white blood cells normally ranges from 4 million to 11 million cells per milliliter of blood.1One teaspoon is made up of about five milliliters. Leukemias are a group of diseases characterized by increased numbers of white cells in the blood and bone marrow.2
In 2023, the American Cancer Society estimates that 59,610 new leukemia cases will be diagnosed and 23,710 people will die from the disease in the United States.3
View a leukemia survivor's perspective: Interview with Tony LaRocco
Please visit the following sections to learn more about leukemia:
- White Blood Cells
- Types of Leukemia
- Risk Factors
- Symptoms
- Detection and Diagnosis
- Leukemia Tumor Biology
- Treatment
- Leukemia Resources
- Section Summary
About White Blood Cells
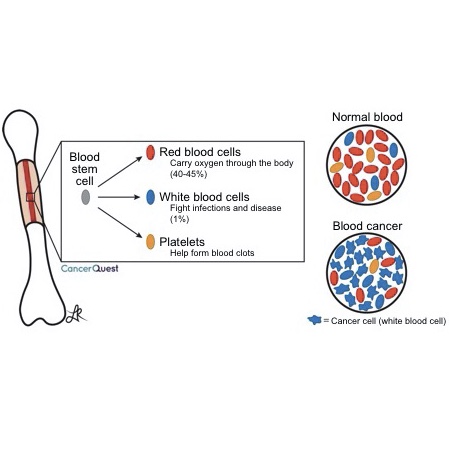
The cells found in blood begin their lives in the middle part (marrow) of some bones. Bone marrow is the source of all blood cells in adults. When cancer arises from one of these cells, the blood no longer has the correct balance of cell types. This can lead to many of the symptoms seen with blood cell cancers. More detailed information is below.
There are five types of leukocytes (white blood cells) found in the blood:
- Basophils
- Eosinophils
- Lymphocytes
- Monocytes
- Neutrophils
Each cell type has a specific role to play in our body's immune system. Important overall functions of the immune system include:
- defense against certain invaders
- removal of dead or damaged cells
- destruction of cancer cells.
Learn more about the immune system
Blood cell formation begins with a special cell located in the bone marrow called a hematopoietic stem cell. Unlike most cells, the hematopoietic stem cell (and stem cells in general) has the capability for self-renewal as well as the ability to give rise to any one of the separate blood cell types. This means that as the stem cell divides in two, one cell replaces the original stem cell and the other begins the multi-step process of developing into a mature blood cell.
It is important to note that hematopoietic stem cells are different from embryonic stem cells. While hematopoietic stem cells can develop into any type of blood cell, embryonic stem cells can develop into any cell type in the entire body.
Blood cell precursors usually progress through a series of stages in the bone marrow before entering the circulating blood stream. Signals from surrounding cells in the bone marrow can help stimulate stem cells to divide as well as develop and mature into specific blood cell types. Normal cell division is also regulated by an important process known as the cell cycle. Disruption of this process is central to the development of leukemia.4Learn more about the cell cycle
Types of Blood Cell Cancers
In the same way that family trees may be divided into bloodlines, the five types of leukocytes (white blood cells) are divided into two groups (lineages), based on how they are developed. The myeloid cells include monocytes, neutrophils, eosinophils and basophils. The lymphoid cells include B lymphocytes, T lymphocytes, and Natural Killer (NK) cells. Learn more about lymphocytes
Leukemias are classified into 4 main categories, based on the type of white blood cell affected (lymphoid vs. myeloid) and characteristics of the disease (acute vs. chronic):
- Acute Lymphoblastic Leukemia
- Acute Myeloid Leukemia (Acute Myelogenous Leukemia)
- Chronic Lymphoid Leukemia (Chronic Lymphocytic Leukemia)
- Chronic Myeloid Leukemia (Chronic Myelogenous Leukemia)
Acute leukemias are generally aggressive diseases in which cancerous transformation occurs at early stages in the development of the affected blood cell. If untreated, these diseases can be rapidly fatal.2
Chronic leukemias are characterized by a slower progression than acute leukemias. These leukemias are more difficult to cure, so the approach to therapy is often conservative and aimed at controlling symptoms.5
Acute Leukemia
Acute leukemias are caused by damage to stem cells or cells in the early stages of development in the bone marrow. Mutations affecting control of cell division, differentiation, and cell death lead to the accumulation of early blood cell precursors known as blast cells.2
Below is a short animation about acute leukemia.
Acute Lymphoblastic Leukemia (ALL)
ALL is the most common form of leukemia diagnosed in children.6 The incidence of ALL peaks between the ages of 3-7, falls by 10 years of age, and rises again after the age of 40.7
Acute Myeloid Leukemia (AML)
AML represents 10-15% of leukemias diagnosed in childhood and is the most common type of acute leukemia diagnosed in adults.8
Chronic lymphoid leukemias are diseases characterized by the accumulation of fully developed B or T lymphocytes in the blood. These diseases are closely related to lymphomas, in which lymphocytes accumulate in lymph nodes and vessels.9
Chronic Lymphocytic Leukemia (CLL)
By far the most common type of chronic lymphoid leukemia involves B lymphocytes. CLL mainly affects elderly individuals, with a peak incidence between 60 and 80 years of age. 9It is the most common form of leukemia in Western countries.10 CLL follows a variable course, with survival ranging from months to decades.10
Other types of chronic lymphoid leukemias include:9
- Prolymphocytic leukemia (PLL)
- Hairy cell leukemia (HCL)
- Plasma cell leukemia
- Large granular lymphocytic leukemia
- T-cell prolymphocytic leukemia (T-PLL)
Chronic Myeloid Leukemia (CML)
CML is a disorder of a hematopoietic stem cell. The disease, which accounts for approximately 15% of leukemias, occurs most frequently between the ages of 40 and 60 years. Laboratory tests reveal increased numbers of cells belonging to the myeloid cell line (monocytes, neutrophils, basophils, eosinophils) at various stages of development circulating in the bloodstream.5
Risk Factors
Although the cause of leukemia in most patients is unknown, several factors are associated with an increased risk of developing the disease. Factors that influence the risk of developing leukemia include:
- Age
- Prior Chemotherapy
- Ethnicity/Gender
- Inherited syndromes (such as Down Syndrome)
- Ionizing Radiation
- Infection by certain viruses
- Cigarette smoking
The relative effects of these and other risk factors in any given case of cancer are variable. Some of these and other risk factors are discussed below.
Age
The risk of developing most types of leukemia increases steadily with age. However, the most common age groups for developing acute lymphoblastic leukemia (ALL) are between the ages of 3-7 and also after the age of 40. 11 The reason for this peak in early childhood ALL remains uncertain.
More information about the relationship between cancer and age can be found in the Mutation section.
Chemotherapy
There is a subset of acute myeloid leukemia (AML), known as "secondary AML" or "therapy-related myeloid leukemia," which can develop following treatment with chemotherapy. Although a causal relationship is implied by the name, the exact mechanism remains unknown.12Prognosis for secondary AML is generally unfavorable compared to primary AML.8
Ethnicity/Gender
With the exception of chronic myeloid leukemia (CML), which has a similar incidence in whites and blacks, leukemia occurs more commonly in those of white ancestry compared to those of Asian, Hispanic and black ancestry. Leukemia also occurs more frequently in males than females.11
Inherited Syndromes
Children with Down syndrome (DS) have a roughly 20-fold increased risk of developing childhood leukemia compared to children without DS.13Approximately 10% of children with DS are born with a "transient leukemia" that resolves spontaneously within months of birth. One to two percent, however, develop a malignant acute leukemia requiring chemotherapy by the age of 4.14 While several hypotheses have been proposed, the reason for this increased risk remains uncertain.
Other inherited syndromes that increase risk of leukemia include:
Ionizing Radiation
An increase in leukemia has been observed in survivors of the atomic bombing of Japanese cities. Although the risk associated with exposure to lower-level radiation is not clear, studies have shown an increase in leukemia following the use of radiotherapy for ankylosing spondylitis (a form of arthritis) and exposure to diagnostic X-rays of the fetus during pregnancy.18
Viruses
Infection with Human T-cell Lymphotropic Virus-1 (HTLV-I) is linked to the development of Adult T-cell Leukemia/Lymphoma (ATLL), a cancer of activated mature T lymphocytes.19Learn more about T lymphocytes
HTLV-I and ATLL are widespread in certain regions of the world, such as the Caribbean basin, Japan, and parts of South America and Africa, while very rare in others.19 Most people who are infected with HTLV-I do not develop leukemia.20 Data from cancer registries in Japan suggest the lifetime risk of developing ATLL among those infected is 2.1% for females and 6.6% for males.21
Although the exact mechanism by which HTLV-I infection induces cancer is not known, laboratory studies have identified several mechanisms which may be involved. 21Learn more about viruses and cancer
Symptoms
Leukemia results in the accumulation of cancer cells in the bone marrow and blood. The presence of large numbers of abnormal cells in the bone marrow can inhibit the marrow from producing normal healthy blood cells. Symptoms caused by bone marrow failure include paleness, tiredness, shortness of breath, excessive bleeding, and increased susceptibility to infections. Cancer cells can also infiltrate organs such as the lymph nodes, spleen, and liver leading to swelling.22
Many patients, however, experience no symptoms at all throughout early stages of the disease.
Watch the full interview with leukemia survivor Tony LaRocco.
Detection And Diagnosis
The diagnosis of leukemia frequenly occurs following a routine blood test that results in an abnormal blood cell count. 23Once leukemia is suspected, the doctor may take samples of bone marrow and blood to examine cell morphology (shape). Samples are also sent to the pathology lab to identify proteins located on the surface and chromosomal and molecular changes within abnormal cells. This information is important for diagnosis, determining the prognosis, and tailoring therapy for individual patients.18
Learn more about cancer detection
Tumor Biology
Genetic changes that occur in cancer include mutation of key regulatory genes, changes in protein products, and changes in the amount of product produced by genes (gene expression). As changes accumulate, cells become more abnormal and cancer progresses. Details of genetic change associated with cancer can be found in the Mutation section.
Advances in leukemia research within recent decades have increased our knowledge about the changes that occur in the disease. A large variety of alterations, including point mutations, amplifications, insertions, deletions, and trisomies are important in development of leukemia. Over 300 chromosomal translocations have been identified so far! A translocation is a rearrangement of parts of a chromosome. Understanding the changes that occur and their effects on cell function allows doctors to classify leukemia into subsets with distinct prognoses and treatment strategies.10
The example of a common translocation, known as the Philadelphia Chromosome, is discussed below. Learn more about translocations
The Philadelphia Chromosome
Translocations involve chromosome breakage and exchange of chromosome fragments. One such translocation, found in over 95% of chronic myeloid leukemias (CML) as well as some acute lymphoblastic leukemias (ALL), occurs between chromosomes 9 and 22. Part of the proto-oncogene abl is removed from chromosome 9 and joined to the bcr gene on chromosome 22. Similarly, part of chromosome 22 is removed and relocated to chromosome 9.24 The exchange leads to the creation of a shortened form of chromosome 22, called the Philadelphia chromosome (after the location of its discovery).
The normal ABL protein functions as a tyrosine kinase. Tyrosine kinases are enzymes that transfer phosphate groups from ATP to other molecules. Activation of key regulatory enzymes in this manner leads to a cascade of events that ultimately results in cell division. The newly created bcr-abl fusion gene located on the Philadelphia chromosome codes for a protein that has increased tyrosine kinase activity, and therefore leads to increased stimulation of cell division, compared to the normal ABL protein.5
Imatinib (Gleevec®) is a drug that was designed to bind to the BCR-ABL fusion protein. The presence of the drug blocks the binding of ATP, preventing the protein from functioning as a tyrosine kinase. 25Imatinib (Gleevec®) is the gold standard treatment for chronic myeloid leukemia (CML).10
Treatment
As our focus is on the biology of the cancers and their treatments, we do not give detailed treatment guidelines. Instead, we link to organizations in the U.S. that generate the treatment guidelines.
The National Cancer Institute lists the following treatments for leukemia:
For more information about how other cancer treatments work, refer to the Cancer Treatments section.
Watch interviews with leukemia survivors Tony LaRocco and Julio Farach (in Spanish)
Information about clinical trials:
- General clinical trial information from CancerQuest
- Click here for information about clinical trials from the National Cancer Institute.
- Click here for information about clinical trials from Georgia Clinical Trials Online.
Leukemia Resources
Risks for Leukemia
Leukemia Risk Factors (Mayo Clinic)
Risk Factors for Childhood Leukemia (ACS)
Detection and Diagnosis of Leukemia
Winship Cancer Institute: Leukemia Cancer Diagnosis and Staging Make an Appointment
What You Need to Know About: Leukemia (NCI)
Leukemia Treatments
Adult Acute Lymphoblastic Leukemia Treatment
Adult Acute Myeloid Leukemia Treatment
Chronic Lymphocytic Leukemia Treatment
Chronic Myelogenous Leukemia Treatment
Childhood Acute Lymphoblastic Leukemia Treatment
Childhood Acute Myeloid Leukemia Treatment
Leukemia Survivorship
Leukemia, Lymphoma Society Report
Long Term Risks for Leukemia Survivors
Long Term ffects of Childhood Leukemia Treatment
International Leukemia Resources
Leukemia and Lymphoma Society of Canada
Canadian Cancer Society: Leukemia
Leukaemia Foundation (Australia)
Cancer Council Australia: Leukaemia
Dharamshila Hospital on Leukemia (India)
Section Summary
Introduction
- The cellular component of blood contains red blood cells, white blood cells, and platelets.
- Leukemia is characterized by an increase in the number of white cells in the blood and bone marrow.
White Blood Cells
- There are five types of white blood cells or leukocytes.
- Leukocytes play an important role in our body's immune system.
- All blood cells form from hematopoietic stem cells.
- Blood cell precursors mature in the bone marrow before entering the circulating bloodstream.
- Disruption of the cell maturation process is central to the development of leukemia.
Types of Leukemia
- Leukemia is divided into 4 main categories based on the affected cell type:
- Acute lymphoblastic or myeloid leukemia (ALL and AML)
- Chronic lymphoid or myeloid leukemia (CLL and CML)
- Acute leukemias are generally aggressive whereas chronic disorders exhibit a slower progression.
Risk Factors
- The risk of developing most types of leukemia increases steadily with age.
- Secondary AML can develop following chemotherapy treatment.
- Leukemia occurs more frequently in white males than in any other population.
- Children with Down syndrome (DS) have a roughly 20-fold increased risk of developing childhood leukemia compared to children without DS.
- Exposure to ionizing radiation increases the risk of leukemia.
- The Human T-cell Leukemia Virus Type 1 (HTLV-1) has been linked to the development of Adult T-cell Leukemia/Lymphoma.
Symptoms
- Paleness, tiredness, shortness of breath, excessive bleeding, and increased susceptibility to infections.
- Swelling of the lymph nodes, spleen, and liver.
Detection and Diagnosis
- Blood and bone marrow tests are used to diagnose leukemia.
Tumor Biology
- Many genetic changes occur in cancer. Details can be found in the Mutation section.
Treatment
- Leukemia treatments include: chemotherapy, immunotherapy, radiation therapy, stem cell transplantation and surgery.
- 1 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 365.
- 2abc Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 157.
- 3 American Cancer Society.Cancer Facts & Figures 2023. Atlanta: American Cancer Society. (2023). 取读于 从 https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html
- 4 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 9.
- 5abc Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 174.
- 6 Stanulla M, Cario G, Meissner B, Schrauder A, Moricke A, Riehm H, Schrappe M. "Integrating molecular information into treatment of childhood acute lymphoblastic leukemia - A perspective from the BFM study group." Blood Cells Mol Dis. 2007 Sep-Oct;39(2):160-3. Epub 2007 May 25. [PUBMED]
- 7 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 158.
- 8ab Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 167.
- 9abc Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 188.
- 10abcd Zhou GB, Li G, Chen SJ, Chen Z. "From dissection of disease pathogenesis to elucidation of mechanisms of targeted therapies: leukemia research in the genomic era." Acta Pharmacol Sin. 2007 Sep;28(9):1434-49. [PUBMED]
- 11ab Yamamoto JF, Goodman MT. "Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002." Cancer Causes Control. 2007 Dec 7 [Epub ahead of print] [PUBMED]
- 12 Larson RA, "Etiology and management of therapy-related myeloid leukemia." Hematology Am Soc Hematol Educ Program. 2007;2007:453-9. [PUBMED]
- 13 Alderton LE, Spector LG, Blair CK, Roesler M, Olshan AF, Robison LL, Ross JA. "Child and maternal household chemical exposure and the risk of acute leukemia in children with Down's syndrome: a report from the Children's Oncology Group." Am J Epidemiol. 2006 Aug 1;164(3):212-21. Epub 2006 Jun 7. [PUBMED]
- 14 Izraeli S, Rainis L, Hertzberg L, Smooha G, Yehudit B. "Trisomy of chromosome 21 in leukemogenesis." Blood Cells Mol Dis. 2007 Sep-Oct;39(2):156-9. Epub 2007 May 29. [PUBMED]
- 15 Armata HL, Garlick DS, Sluss HK. "The ataxia telangiectasia-mutated target site ser18 is required for p53-mediated tumor suppression." Cancer Res. 2007 Dec 15;67(24):11696-703. [PUBMED]
- 16 Meyer S, Fergusson WD, Whetton AD, Moreira-Leite F, Pepper SD, Miller C, Saunders EK, White DJ, Will AM, Eden T, Ikeda H, Ullmann R, Tuerkmen S, Gerlach A, Klopocki E, Tonnies H. "Amplification and translocation of 3q26 with overexpression of EVII in Fanconi anemia-derived childhood acute myeloid leukemia with biallelic FANCDI/BRCA2 disruption." Genes Chromosomes Cancer. 2007 Apr;46(4):359-72. [PUBMED]
- 17 Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA. "Cancer incidence and mortality in men with klinefelter syndrome: a cohort study." J Natl Cancer Inst. 2005 Aug 17;97(16):1204-10. [PUBMED]
- 18ab Boon et al. "Principles & Practice of Medicine." (20th edition) Churchill Livingstone, 2006. Pgs 1039-1047.
- 19ab Matutes E. "Adult T-cell leukaemia/lymphoma." J Clin Pathol. 2007 Dec;60(12):1373-7. [PUBMED]
- 20 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 195.
- 21ab Taylor G. "Molecular aspects of HTLV-1 infection and adult T-cell leukaemia/lymphoma." J Clin Pathol. 2007 Dec;60(12):1392-6. [PUBMED]
- 22 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 159.
- 23 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 189.
- 24 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 180.
- 25 Hoffbrand AV, Moss PAH, Pettit JE (ed). "Essential Haematology" 5th Edition. Blackwell Publishing, Oxford: 2006. Pg. 155.
