Kidney cancer is one of the 10 most common cancers to affect men and women. It is more common in older people. The average age of those diagnosed with kidney cancer is 64 years old. The American Cancer Society (ACS) estimates that in 2023, approximately 81,800 people will be diagnosed with kidney cancer in the U.S. Approximately 14,890 people are predicted to die from cancer of the kidney and renal pelvis in 2023.1
Please visit the following sections to learn more about kidney cancer:
- Types of kidney cancer
- Anatomy and function of the kidneys
- Risk factors
- Prevention
- Symptoms
- Detection and diagnosis
- Cancer staging
- Tumor biology
- Treatment
- Resources
- Kidney cancer section summary
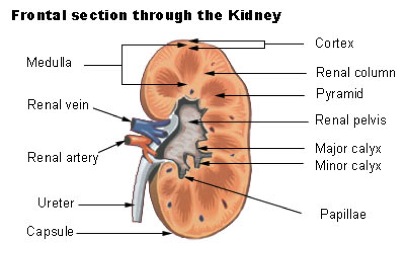
Anatomy And Function Of The Kidneys
Kidneys are fist-sized organs that are part of the urinary system. Everyone has 2 kidneys, which are located on either side of the spine, right below the ribcage. Each kidney is made of millions of filtering units known as nephrons. Each nephron is made of a glomerular (Bowman's) capsule and a renal tubule, and is located in the regions of the kidney known as the renal cortex and renal medulla. Specific information regarding these two kidney regions can be found below. Blood is initially filtered in the glomerular capsule to form urine. The renal tubule further processes the urine by allowing water and ions to be added to or taken out of the urine.2
The kidneys are responsible for blood filtration. In a day's time, the two kidneys are able to filter approximately 48 gallons of blood. The material filtered out of the blood by the kidneys is used to produce urine. The kidneys help to eliminate the buildup of potentially harmful chemicals by causing them to be released from the body in urine.2
Each kidney can be divided into 2 major regions. These two regions work together to produce and filter urine for excretion out of the body.
- Renal cortex: This is the outer portion of the kidney that consists of the glomerular capsule and a portion of the renal tubule (cortical collecting, duct, proximal and distal convoluted tubules). The structures in this region of the kidney are responsible for filtering the fluid that flows through. Unwanted materials are secreted into urine and necessary water and nutrients are reabsorbed back into the body.3
- Renal medulla: This is the inner portion of the kidney that consists of the remaining portions of the renal tubule (loop of Henle and medullary collecting duct). This region of the kidney is responsible for concentrating urine for excretion out of the body. Salt levels (osmolarity) are higher towards the inner renal medulla, so as urine descends through the collecting duct towards the inner renal medulla, water is reabsorbed back into the tissues, concentrating the urine.3
From left to right: Frontal Section of a Kidney, Kidney Nephron
Types
In general, tumors can be cancerous (malignant) or non-cancerous (benign). Kidney cancer refers to malignant tumors, originating in the kidneys, which can spread (metastasize) to other places in the body.
The main type of kidney cancer is renal cell carcinoma (RCC), which is responsible for approximately 90% of all kidney cancer cases, 2.4% of all adult cancer cases, and over 140,000 deaths annually.4 RCC can be categorized into several subtypes, depending on the structure and function of the affected cells.
- Clear cell RCC: This is the most common type of RCC. Approximately 70% of people with RCC have this kind of cancer. The cells in this subtype appear very pale or clear.4
- Papillary RCC: Approximately 10% of people with RCC have this kind of cancer. The cells in this subtype form finger-like projections in most of the tumor.5
- Chromophobe RCC: Approximately 5% of people with RCC have this kind of cancer. The cells in this subtype also appear very pale or clear but are much larger in size than the cells of clear cell RCC.5
- Collecting duct RCC, multilocular cystic RCC, medullary carcinoma, mucinous tubular and spindle cell carcinoma, and neuroblastoma-associated RCC are rare RCC subtypes that, together, make up less than 1% of all RCC cases.6
The remaining 10% of kidney cancers include the following:
- Transitional cell carcinomas (TCCs) or urothelial carcinomas: Approximately 5-10% of people with kidney cancer have this kind of cancer. TCCs originate in the lining of the renal pelvis; a funnel-like structure in the kidneys that collects and drains urine.5
- Wilms tumors (Nephroblastoma): This kind of kidney cancer is very rare in adults; the majority of all Wilms tumor cases are in children.5 Wilms tumors account for approximately 5% of all childhood cancer cases and approximately 1.1% of all kidney cancer cases.5, 7 The majority of Wilms tumor cases occur in children between the ages of two and three.8
More information regarding the possible link between Wilms tumor and genetic changes (mutations) in the WT1 gene can be found on CancerQuest's page on kidney cancer risk factors and kidney cancer tumor biology.
- Renal sarcomas: Less than 1% of people with kidney cancer have this kind. Renal sarcomas originate in the blood vessels or connective tissues of the kidney.6
Risk Factors
The direct cause of kidney cancer in any specific individual is currently unknown. Studies indicate higher rates of renal cell cancer (RCC) among men than among women. The rates also vary geographically; higher rates are found in Europe and North American than in Asia and South America. It has also been shown that RCC cases are being diagnosed at an earlier stage than in the past, which could be accounted for by better imaging technology that reveals earlier stage cancers. The following is a list of some of the known risk factors for developing kidney cancer. These risk factors are expanded upon, below. Note that having a risk factor does not necessarily mean an individual will develop kidney cancer.5
- Cigarette smoking
- Obesity
- Hypertension
- Genetic predisposition
Cigarette Smoking
Men who smoke have a 50% greater risk of developing renal cell cancer (RCC) than men who have never smoked. Women who smoke have a 20% greater risk of developing RCC than women who have never smoked. Cigarette smoke contains cancer-causing (carcinogenic) chemicals, including N-nitrosamines that damage the DNA in cells, including kidney cells.5
For more information about tobacco's role in cancer development, visit CancerQuest's page on tobacco.
Obesity
Studies indicate that overweight and baseline-obese individuals increase their risk for developing RCC by 24% (men) and 34% (women), for every 5 kg/m2 increase (roughly 0.007 pounds/in2) in body mass index (BMI). BMI is measured based on an individual's height and weight. A person with a higher BMI has a higher body fat level.5
Hypertension
High blood pressure (hypertension) is a chronic disease that, if left uncontrolled, can damage kidney functions and lead to kidney diseases. Studies suggest that people with hypertension may be at increased risk for RCC.5
Genetic Predisposition
Most cases of RCC are not thought to be due to heredity. However, individuals who have the following genetic conditions are at an increased risk for developing RCC:
- Von Hippel-Lindau syndrome (VHL) [see description here]
- Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC)
- Birt-Hogg-Dubésyndrome (BHD)
- Hereditary Papillary Renal Cancer (HPRC).9, 10
Studies suggest that individuals with CTR9 gene mutations are predisposed to Wilms tumor.11 The children and grandchildren of parents with Wilms are at risk for developing Wilms tumor. It is recommended that children of a parent who had Wilms tumor is both kidneys (bilateral Wilms tumor) undergo ultrasound screenings every 3 months until they are 8 years of age.12 Children with WAGR syndrome (Wilms tumor, aniridia, genitourinary anomalies, and mental retardation) have a greater than 30% risk for developing Wilms tumor.12
In some children, aniridia (eye disease involving absence of the iris) may be a result of large chromosomal deletions that also include the WT1 gene. Because of this, those children are 67 times more likely to develop Wilms tumors than children without those large chromosomal deletions.13 Children who have sporadic aniridia and a normal WT1 gene are not at an increased risk for developing Wilms tumor.14
More on the genetics of kidney cancer.
Evidences also suggest possible associations between the following risk factors and RCC development, but are not conclusive:
- Diabetes mellitus
- Alcohol consumption
- Exposure to trichloroethylene, such as in factories where trichloroethylene is used. The following are examples of general household products that may contain trichloroethylene: rust removers, sealants, degreasers, fixatives, and cleaners15
- Parity in women (parity refers to the number of times a pregnant woman has carried a fetus to a viable gestational age)
Prevention
Although the direct cause of kidney cancer in an individual is currently unknown, certain factors may increase an individual's risk for developing kidney cancer. The following are lifestyle choices that may help lower these risks:6, 16
- Quitting smoking
- Maintaining healthy weight
- Controlling high blood pressure
- Eating more fruits and vegetables
- Avoiding occupational exposure to heavy metals, such as cadmium and lead. The following are examples of occupational exposure to cadmium and lead: welding or soldering cadmium plated material (e.g. aircraft and electronics industries), manufacturing cadmium-tin welding rods, working in casting areas, manufacturing ammunition, spraying lead-based paints, and melting lead.17
Symptoms
Early stage kidney cancer typically does not produce symptoms. Larger kidney cancer tumors and kidney cancers that have spread (metastasized) to other parts of the body are more likely to produce symptoms. The symptoms listed below are associated with kidney cancer but may also be due to other conditions.6
- Anemia
- Blood in urine (hematuria)
- Fatigue
- Loss of appetite
- Lump or pain in the lower back
- Weight loss
Detection And Diagnosis
If an individual suspects he/she may have kidney cancer, he/she should consult a urologic oncologist (a doctor who specializes in the treatment of urinary tract cancers). The doctor will ask the patient about his/her health history and may choose to conduct further tests to find out more information. The following are some examples of tests that the oncologist may perform.6, 18
- Physical exams
During physical exams, doctors will feel (palpate) for growths and indications of kidney tumors. Due to the kidneys’ deep location inside the body, small kidney tumors may not be felt during a physical exam.
- CT scans
CT scans are a type of X-ray that provides detailed information regarding a tumor’s shape, size, and position, making it one of the most useful imaging tests in kidney cancer detection.
For more information, visit CancerQuest's page on CT scans.
- Ultrasound
An ultrasound uses sound waves to detect masses on the kidneys and to show whether the identified mass is solid or fluid-filled.
For more information, visit CancerQuest's page on ultrasounds.
- PET scans
Positron Emission Tomography (PET) scans are used to track the movement of tagged chemicals in the body. PET images are not as detailed as CT scan images and cannot provide precise information about a tumor's shape, size, and position. PET scans can be used to identify the general location of a tumor and the location(s) of where the cancer has spread (metastasized).
For more information, visit CancerQuest's page on PET scans.
PET Scan Machine. Image courtesy of the NCI
- Lab tests
The doctor may choose to perform blood tests to measure a patient’s red blood cell, white blood cell, and platelet levels. Patients who have kidney cancer tend to have fewer red blood cells.
The doctor may also choose to perform urinalysis, which is a type of test where a patient’s urine sample is examined for traces of blood and other substances. Abnormal urinalysis results may indicate kidney problems. The doctor may also choose to examine the urine for cancer cells (urine cytology).
For additional information on cancer detection and diagnosis tests, visit CancerQuest's page on cancer detection and diagnosis.
Staging
Staging allows doctors to figure out the degree to which the cancer has spread (metastasized) so that they will be able to work with the patient to develop a treatment plan. Staging is most often done during surgery, where a surgical oncologist takes tissue samples from targeted regions, for analysis.
The most common staging system used for kidney cancer is T/N/M staging. This system categorizes kidney cancer based on the size and location of the cancer. For information on T/N/M staging, visit CancerQuest's page on T/N/M staging. Some doctors choose to use the UCLA Integrated Staging System, which takes into account the TNM staging as well as other factors, including a patient's health history and the tumor's Fuhrman grade. The Fuhrman grade reflects how different the cancerous kidney cell nuclei are from healthy kidney cell nuclei.6
The following is a general summary of the stages of kidney cancer.
- Stage I: The cancerous tumor is found only in the kidneys; it has not spread (metastasized) to lymph nodes or other organs. The cancerous tumor is equal to or less than 7cm (2.8in) across.
- Stage II: The cancerous tumor is found only in the kidneys; it has not spread (metastasized) to lymph nodes or other organs. The cancerous tumor is greater than 7cm across.
- Stage III: The cancer cells have grown into major veins (i.e. renal vein or vena cava) and may have spread (metastasized) to nearby lymph nodes. The cancer cells have not spread to distant lymph nodes or other organs.
- Stage IV: This is the most advanced stage of kidney cancer. Cancer cells have spread (metastasized) beyond the renal fascia (Gerota's fascia) and may/may not have spread to nearby/distant lymph nodes and organs.
Tumor Biology
The molecular basis of kidney cancer is currently poorly understood. However, in recent years, studies have identified several genes that are linked to the development and spread of renal cell carcinoma (RCC), the most prevalent form of kidney cancer. RCC accounts for approximately 90% of all kidney cancer cases. Identification of these genes has been crucial in the development of drugs to treat the disease. The following is a list that includes some of the genes and pathways involved in kidney cancer development.
- Von Hippel-Lindau (VHL) tumor suppressor gene: This gene encodes the VHL protein, which is a tumor suppressor that can eliminate a protein called HIF (hypoxia-inducible factor). HIF turns on and off genes and changes cell behaviors. 19, 20 Without functional VHL protein, HIF1 is active and cells continue to grow and divide abnormally, leading to tumor formations. Studies have found that VHL gene mutations are linked to clear cell RCC development and can either spontaneously arise or be inherited.21, 22 Researchers evaluating VHL gene expression in RCC patients observed VHL gene mutations in 95.23% of clear cell RCC patients.23
- VEGF gene: This gene encodes the vascular endothelial growth factor (VEGF), which is a key protein involved in blood vessel formation (angiogenesis) and vascular permeability.20 Studies show that VEGF is commonly overexpressed in RCC.24
- Polybromo1 (PBRM1) gene: This gene codes for the BAF180 protein, which is a subunit of the SWI/SNF chromatin remodeling complex. (5969) A study found that 41% (92/227) of its clear cell RCC patients had PBRM1 gene mutations. Furthermore, 13 out of 14 patients without VHL gene mutations still had PBRM1 gene mutations, suggesting the importance of PBRM1 in typical clear cell RCC development.25
- BRCA related protein-1 (BAP1) gene: This gene encodes for the BAP1 protein, which functions as a nuclear deubiquitinase. A study found that, in vitro, without functional BAP1 protein, RCC cells become sensitive to DNA damage (genotoxic stress). BAP1 was also shown to be inactivated in 15% of clear cell RCCs.26
- TP53 gene: This gene encodes for the tumor suppressor protein, p53, which is in charge of monitoring cell division and cell death (apoptosis). Approximately half of all human tumors have a mutation in the TP53 tumor suppressor gene. Although p53's role is very clear in some cancer types, its exact role in RCC is still relatively uncertain. There are ongoing studies aimed at figuring out the mechanisms by which p53 acts, specifically in RCC.27, 28, 4
- WT1 gene: This gene encodes for a transcription factor that is involved in the transcription regulation of a number genes. These target genes encode for proteins, such as growth factors, growth factor receptors, transcription factors, and other proteins. Studies have found that WT1 can also act as a tumor suppressor in clear cell RCC. Studies found significantly lower levels of WT1 RNA in clear cell RCC samples compared with tumor-free renal tissue.29, 30, 31 Deletion of WT1 gene increases the risk for Wilms tumor.12WT1 gene mutations account for approximately 10-20% of Wilms tumor cases.32
- CTR9 gene: This gene encodes for a component of the RNA polymerase II complex, which is involved in regulation of transcription. Studies suggest CTR9 acts as a tumor suppressor gene and mutations in CTR9 may increase risk for Wilms tumor.11
- WNT/B-catenin pathway, IGF2 pathway, and p53 pathway: Studies have found that these three pathways are disrupted in Wilms tumor (nephroblastoma) development.8
- PI3K/AKT/mTOR pathway: Studies have found that this pathway is recurrently mutated in clear cell RCC. This suggests that the pathway may be a possible therapeutic target.33, 34, 4
In addition to studies on genetic mutations, epigenetic studies have suggested a possible correlation between DNA methylation of gremlin1 and poorer overall survival rates in clear cell RCC patients, as well as a possible correlation between DNA methylation of the GATA5 with increased metastasis.35, 36 Studies are currently ongoing in these areas, and there is no evidence that conclusively implicates these methylation events in kidney cancer development or spread.
For more information, visit CancerQuest's page on DNA methylation and epigenetic changes in cancer.
Treatment
Treatment options for kidney cancer depend on the tumor's location, the degree to which the tumor is affecting kidney functions, and the patient's health history. Some treatment options include chemotherapy, radiation therapy, surgery, and biological therapy.
Since CancerQuest's focus is on the cancer's biology and the biology of possible treatments, we do not give detailed treatment guidelines. Instead, we link to organizations in the U.S. that do generate treatment guidelines:
- Learn about the treatments recommended by the National Comprehensive Cancer Network (NCCN) for Kidney Cancers.
Learn about how cancer treatments work at CancerQuest's page on cancer treatments.
For information about clinical trials:
- Information about clinical trials from CancerQuest
- Information about clinical trials from the National Cancer Institute
- Information about clinical trials from Georgia Clinical Trials Online
Kidney Cancer Resources
Risks for Kidney Cancer
Kidney Cancer Risk Factors (Mayo Clinic)
Risk Factors: Kidney Cancer (ACS)
Detection and Diagnosis of Kidney Cancer
Cancer Topic: Kidney Cancer (NCI)
Wilms Tumor and Other Childhood Kidney Tumors
Kidney Cancer: What You Need To Know
Detailed Guide to Kidney Cancer (ACS)
Overview Guide to Kidney Cancer (ACS)
Kidney Cancer Treatments
Winship Cancer Institute: Kidney Cancer
Renal Cell Cancer Treatment (NCI)
Transitional Cell Cancer Renal/Ureter Treatment (NCI)
Wilms Tumor and Other Childhood Kidney Tumors Treatment (NCI)
Kidney Cancer Survivorship
Long Term Risks for Kidney Cancer Survivors
Surgery for Kidney Cancer (ACS)
International Kidney Cancer Resources
Kidney Cancer (Cancer Council Australia)
Kidney Cancer (Cancer Research UK)
Section Summary
Introduction
- Kidney cancer is one of the 10 most common cancers to affect men and women.
- The average age of those diagnosed with kidney cancer is 64 years old.
Types of Kidney Cancer
- The most common type of kidney cancer is renal cell carcinoma (RCC), which is responsible for approximately 90% of all kidney cancer cases. RCC can be categorized into several subtypes: clear cell RCC, papillary RCC, chromophobe RCC, and more.
- The remaining 10% of kidney cancers include the following: transitional cell carcinomas, Wilms tumors, and renal sarcomas.
Risk Factors
- Smoking increases the risk of developing RCC in men and women.
- Obesity is linked to an increased risk for developing RCC in men and women.
- Children with WT1 gene mutations are at an increased risk for developing Wilms tumor.
Symptoms and Detection
- Early stages of kidney cancer typically do not produce symptoms.
- Several medical tests can be used to detect or rule out a kidney tumor. Examples include: physical exams, CT scans, PET scans, and ultrasound scans.
Staging and Pathology
- The T/N/M system is one of the most common methods used for kidney cancer staging.
- The T/N/M system assigns a degree of severity based on the size and location of the cancer.
Treatment
- Treatments differ depending on specific factors, such as the patient's age, patient's health conditions, cancer stage, tumor's location, and more.
- Treatments can include surgery, radiation therapy, chemotherapy, and biological therapy.
- 1 American Cancer Society.Cancer Facts & Figures 2023. Atlanta: American Cancer Society. (2023). 取读于 从 https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html
- 2ab Ogobuiro, I., & Tuma, F. (2019). Physiology, Renal.. FL: Treasure Island: StatPearls Publishing. [PUBMED]
- 3ab Arthur JM, Thongboonkerd V, Scherzer JA, Cai J, Pierce WM, Klein JB. Differential expression of proteins in renal cortex and medulla: a proteomic approach. Kidney Int. 2002 Oct;62(4):1314-21. [PUBMED]
- 4abcd Scelo G, Riazalhosseini Y, Greger L, Letourneau L, Gonzàlez-Porta M, Wozniak MB, Bourgey M, Harnden P, Egevad L, Jackson SM, Karimzadeh M, Arseneault M, Lepage P, How-Kit A, Daunay A, Renault V, Blanché H, Tubacher E, Sehmoun J, Viksna J, Celms E, Opmanis M, Zarins A, Vasudev NS, Seywright M, Abedi-Ardekani B, Carreira C, Selby PJ, Cartledge JJ, Byrnes G, Zavadil J, Su J, Holcatova I, Brisuda A, Zaridze D, Moukeria A, Foretova L, Navratilova M, Mates D, Jinga V, Artemov A, Nedoluzhko A, Mazur A, Rastorguev S, Boulygina E, Heath S, Gut M, Bihoreau MT, Lechner D, Foglio M, Gut IG, Skryabin K, Prokhortchouk E, Cambon-Thomsen A, Rung J, Bourque G, Brennan P, Tost J, Banks RE, Brazma A, Lathrop GM. Variation in genomic landscape of clear cell renal cell carcinoma across Europe. Nat Commun. 2014 Oct 29;5:5135. doi: 10.1038/ncomms6135. [PUBMED]
- 5abcdefghi Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010 May;7(5):245-57. doi: 10.1038/nrurol.2010.46. [PUBMED]
- 6abcdef ACS 2014 Kidney Cancer Detailed Guide [http://www.cancerquest.org/sites/default/files/assets/pdf/Kidney/kidney-cancer-detailed-guide-acs.pdf]
- 7 What are the key statistics about Wilms tumor? American Cancer Society. [http://www.cancer.org/cancer/wilmstumor/detailedguide/wilms-tumor-key-statistics]
- 8ab Maschietto, M., Charlton, J., Perotti, D., Radice, P., Geller, J., Pritchard-Jones, K., & Weeks, M. (2014). The IGF signalling pathway in Wilms tumours--a report from the ENCCA Renal Tumours Biology-driven drug development workshop. Oncotarget, 5(18), 8014–26. (Original work published 2014年9月) [PUBMED]
- 9 Genetics of Kidney Cancer (Renal Cell Cancer). National Cancer Institute at the National Institutes of Health. U.S. Department of Health and Human Services. [http://www.cancer.gov/cancertopics/pdq/genetics/kidney/HealthProfessional/page1]
- 10 Major Heritable Renal Cell Cancer Syndromes. National Cancer Institute at the National Institutes of Health. U.S. Department of Health and Human Services. [http://www.cancer.gov/cancertopics/pdq/genetics/kidney/HealthProfessional/page2]
- 11ab Hanks S, Perdeaux ER, Seal S, Ruark E, Mahamdallie SS, Murray A, Ramsay E, Del Vecchio Duarte S, Zachariou A, de Souza B, Warren-Perry M, Elliott A, Davidson A, Price H, Stiller C, Pritchard-Jones K, Rahman N. Germline mutations in the PAF1 complex gene CTR9 predispose to Wilms tumour. Nat Commun. 2014 Aug 7;5:4398. doi: 10.1038/ncomms5398. [PUBMED]
- 12abc Wilms Tumor and Other Childhood Kidney Tumors Treatment. National Cancer Institute at the National Institutes of Health. U.S. Department of Health and Human Services. [http://www.cancer.gov/cancertopics/pdq/treatment/wilms/HealthProfessional/page2]
- 13 Gronskov K, Olsen JH, Sand A, Pedersen W, Carlsen N, Bak Jylling AM, Lyngbye T, Brondum-Nielsen K, Rosenberg T. Population-based risk estimates of Wilms tumor in sporadic aniridia. A comprehensive mutation screening procedure of PAX6 identifies 80% of mutations in aniridia. Hum Genet. 2001 Jul;109(1):11-8. [PUBMED]
- 14 Scott RH, Walker L, Olsen OE, Levitt G, Kenney I, Maher E, Owens CM, Pritchard-Jones K, Craft A, Rahman N. Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch Dis Child. 2006 Dec;91(12):995-9. Epub 2006 Jul 20. [PUBMED]
- 15 Trichloroethylene. Household Products Database. U.S. Department of Health and Human Services. [http://hpd.nlm.nih.gov/cgi-bin/household/search?tbl=TblChemicals&queryx=79-01-6]
- 16 Kidney Cancer Prevention. Mayo Clinic. [http://www.mayoclinic.org/diseases-conditions/kidney-cancer/basics/prevention/con-20024753]
- 17 Boffetta P, Fontana L, Stewart P, Zaridze D, Szeszenia-Dabrowska N, Janout V, Bencko V, Foretova L, Jinga V, Matveev V, Kollarova H, Ferro G, Chow WH, Rothman N, van Bemmel D, Karami S, Brennan P, Moore LE. Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: a case-control study from Central and Eastern Europe. Occup Environ Med. 2011 Oct;68(10):723-8. doi: 10.1136/oem.2010.056341. Epub 2011 Jan 8. [PUBMED]
- 18 ACS 2014 Kidney Cancer Overview Guide [/sites/default/files/assets/pdf/Kidney/kidney-cancer-overview-guide-acs.pdf]
- 19 VHL. Genetics Home Reference. National Institutes of Health. U.S. Department of Health and Human Services. Reviewed August 2012, Published August 2015 [http://ghr.nlm.nih.gov/gene/VHL]
- 20ab Zhuang J, Tu X, Cao K, Guo S, Mao X, Pan J, Huang B, Chen X, Gao Y, Qiu S. The expression and role of tyrosine kinase ETK/BMX in renal cell carcinoma. J Exp Clin Cancer Res. 2014 Mar 7;33:25. doi: 10.1186/1756-9966-33-25. [PUBMED]
- 21 Cowey CL, Rathmell WK. VHL gene mutations in renal cell carcinoma: role as a biomarker of disease outcome and drug efficacy. Curr Oncol Rep. 2009 Mar;11(2):94-101. [PUBMED]
- 22 Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, de Bruine AP. VHL and HIF signalling in renal cell carcinogenesis. J Pathol. 2010 Jun;221(2):125-38. doi: 10.1002/path.2689. [PUBMED]
- 23 Shahzad H, Kehar SI, Ali S, Tariq N. Expression of Von Hippel - Lindau (VHL) gene mutation in diagnosed cases of renal cell carcinoma. Pak J Med Sci. 2014 Jul;30(4):880-5. [PUBMED]
- 24 Choueiri TK, Fay AP, Gagnon R, Lin Y, Bahamon B, Brown V, Rosenberg JE, Hutson TE, Baker-Neblett KL, Carpenter C, Liu Y, Pandite L, Signoretti S. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res. 2013 Sep 15;19(18):5218-26. doi: 10.1158/1078-0432.CCR-13-0491. Epub 2013 Jul 23. [PUBMED]
- 25 Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin ML, Teague J, Bignell G, Butler A, Cho J, Dalgliesh GL, Galappaththige D, Greenman C, Hardy C, Jia M, Latimer C, Lau KW, Marshall J, McLaren S, Menzies A, Mudie L, Stebbings L, Largaespada DA, Wessels LF, Richard S, Kahnoski RJ, Anema J, Tuveson DA, Perez-Mancera PA, Mustonen V, Fischer A, Adams DJ, Rust A, Chan-on W, Subimerb C, Dykema K, Furge K, Campbell PJ, Teh BT, Stratton MR, Futreal PA. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011 Jan 27;469(7331):539-42. doi: 10.1038/nature09639. Epub 2011 Jan 19. [PUBMED]
- 26 Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, Kinch L, Hambuch T, Jain S, Lotan Y, Margulis V, Sagalowsky AI, Summerour PB, Kabbani W, Wong SW, Grishin N, Laurent M, Xie XJ, Haudenschild CD, Ross MT, Bentley DR, Kapur P, Brugarolas J. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012 Jun 10;44(7):751-9. doi: 10.1038/ng.2323. [PUBMED]
- 27 Zigeuner R, Ratschek M, Rehak P, Schips L, Langner C. Value of p53 as a prognostic marker in histologic subtypes of renal cell carcinoma: a systematic analysis of primary and metastatic tumor tissue. Urology. 2004 Apr;63(4):651-5. [PUBMED]
- 28 Villaamil VM, Gallego GA, Caínzos IS, Ruvira LV, Valladares-Ayerbes M, Aparicio LM. Relevant Networks involving the p53 Signalling Pathway in Renal Cell Carcinoma. Int J Biomed Sci. 2011 Dec;7(4):273-82. [PUBMED]
- 29 Sitaram RT, Degerman S, Ljungberg B, Andersson E, Oji Y, Sugiyama H, Roos G, Li A. Wilms' tumour 1 can suppress hTERT gene expression and telomerase activity in clear cell renal cell carcinoma via multiple pathways. Br J Cancer. 2010 Oct 12;103(8):1255-62. doi: 10.1038/sj.bjc.6605878. Epub 2010 Sep 14. [PUBMED]
- 30 Niu Z, Ito M, Awakura Y, Takahashi T, Nakamura E, Ito N, Ogawa O. The expression of NOV and WT1 in renal cell carcinoma: a quantitative reverse transcriptase-polymerase chain reaction analysis. J Urol. 2005 Oct;174(4 Pt 1):1460-2. [PUBMED]
- 31 Nakatsuka S, Oji Y, Horiuchi T, Kanda T, Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M, Kitamura Y, Oka Y, Kawase I, Sugiyama H, Aozasa K. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol. 2006 Jun;19(6):804-14. [PUBMED]
- 32 WT1. Genetics Home Reference. Lister Hill National Center for Biomedical Communications. U.S. National Library of Medicine. National Institutes of Health. U.S. Department of Health and Human Services. [http://ghr.nlm.nih.gov/gene/WT1]
- 33 Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013 Jul 4;499(7456):43-9. doi: 10.1038/nature12222. Epub 2013 Jun 23. [PUBMED]
- 34 Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki H, Nagata Y, Yoshida K, Kon A, Suzuki Y, Chiba K, Tanaka H, Niida A, Fujimoto A, Tsunoda T, Morikawa T, Maeda D, Kume H, Sugano S, Fukayama M, Aburatani H, Sanada M, Miyano S, Homma Y, Ogawa S. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013 Aug;45(8):860-7. doi: 10.1038/ng.2699. Epub 2013 Jun 24. [PUBMED]
- 35 van Vlodrop IJ, Baldewijns MM, Smits KM, Schouten LJ, van Neste L, van Criekinge W, van Poppel H, Lerut E, Schuebel KE, Ahuja N, Herman JG, de Bruine AP, van Engeland M. Prognostic significance of Gremlin1 (GREM1) promoter CpG island hypermethylation in clear cell renal cell carcinoma.Am J Pathol. 2010 Feb;176(2):575-84. doi: 10.2353/ajpath.2010.090442. Epub 2009 Dec 30. [PUBMED]
- 36 Peters I, Eggers H, Atschekzei F, Hennenlotter J, Waalkes S, Trankenschuh W, Grosshennig A, Merseburger AS, Stenzl A, Kuczyk MA, Serth J. GATA5 CpG island methylation in renal cell cancer: a potential biomarker for metastasis and disease progression. BJU Int. 2012 Jul;110(2 Pt 2):E144-52. doi: 10.1111/j.1464-410X.2011.10862.x. Epub 2012 Jan 30. [PUBMED]